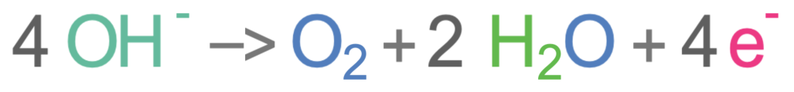C4 K) Electrolysis – Part 2
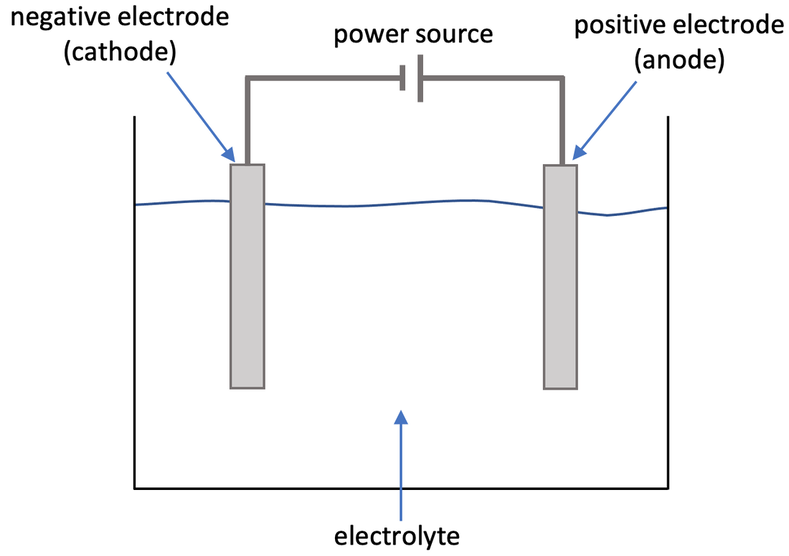
Electrolysis is the splitting up of an ionic compound by using electricity. The general setup for electrolysis is shown below.
The electrolyte is a molten form of the ionic compound or the ionic compound dissolved in water (an aqueous solution). The electrolyte needs to be molten or an aqueous solution so that the ions in the ionic compound are free to move and conduct electricity. The electrolyte cannot be a solid ionic compound because the ions in a solid ionic compound are in their fixed positions and are unable to move, which means that they will not conduct electricity.
In the examples in the previous section, we looked at electrolysis where the electrolyte was a molten ionic substance. In this section, we are going to look at electrolysis where the electrolyte is an aqueous solution (the ionic compound will be dissolved in water).
In an aqueous solution, we will get ions from both the ionic compound and the water; the water will give us positive hydrogen ions (H+ ions) and negative hydroxide ions (OH-). The equation for water giving us these ions is shown below:
This means that there will be four different types of ions in the electrolyte. These ions are the positive metal ions and negative non-metal ions from the ionic compound, and the positive hydrogen ions and negative hydroxide ions from the water.
Cathode (Negative Electrode)
What happens at the cathode in an aqueous solution depends on the positive hydrogen ions (H+) and the positive metal ions present.
If the metal is more reactive than hydrogen, the positive hydrogen ions will be attracted to the cathode and hydrogen gas will be produced (H2 gas will be produced).
However, if the metal is less reactive than hydrogen, the positive metal ions will be attracted to the cathode, which results in a layer of the less reactive metal appearing around the cathode.
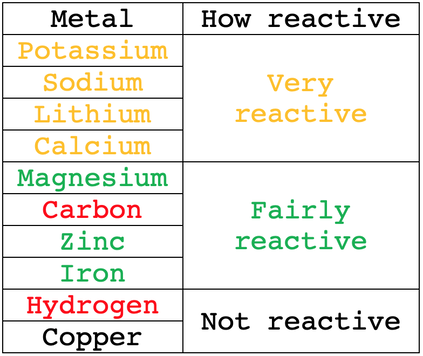
The reactivity series for the metals and hydrogen is shown below.
From the reactivity series, we can see that hydrogen is only more reactive than copper. This means that if the electrolyte was a solution of copper sulfate, the less reactive positive copper ions would be attracted to the cathode, and the more reactive positive hydrogen ions will stay in the aqueous solution (we will be looking at this example in more detail later on).
For the metals that are more reactive than hydrogen, the positive metal ions will stay in the electrolyte, and the positive hydrogen ions will be attracted to the cathode where they will produce hydrogen gas (H2). For example, let’s have a look at what would happen if the electrolyte was an aqueous solution of sodium chloride. Sodium is more reactive than hydrogen. This means that the positive hydrogen ions (H+) will be attracted to the cathode, and hydrogen gas will be produced (H2). The more reactive sodium ions will stay in the electrolyte (we will be looking at this example in a bit more detail later).
Anode (Positive Electrode)
In aqueous electrolytes, there will always be negative hydroxide ions present (OH-).
The rules at the anode depend on whether halide ions are present; halide ions are negative halogen ions and they include chloride (Cl-), bromide (Br-) and iodide (I-).
If halide ions are present, molecules of the halogen will be produced at the anode. For example, if chloride ions are present in the electrolyte, chlorine molecules will be produced at the anode; Cl2 will be produced at the anode. All of the halogen molecules produced at the anode when halide ions are present will be diatomic molecules (they will be pairs; Cl2, Br2 and I2).
If no halide ions are present, the negative hydroxide ions will form oxygen and water at the anode. The oxygen will bubble out of the aqueous electrolyte into the air in the lab.
Let’s now have a look at a few examples.
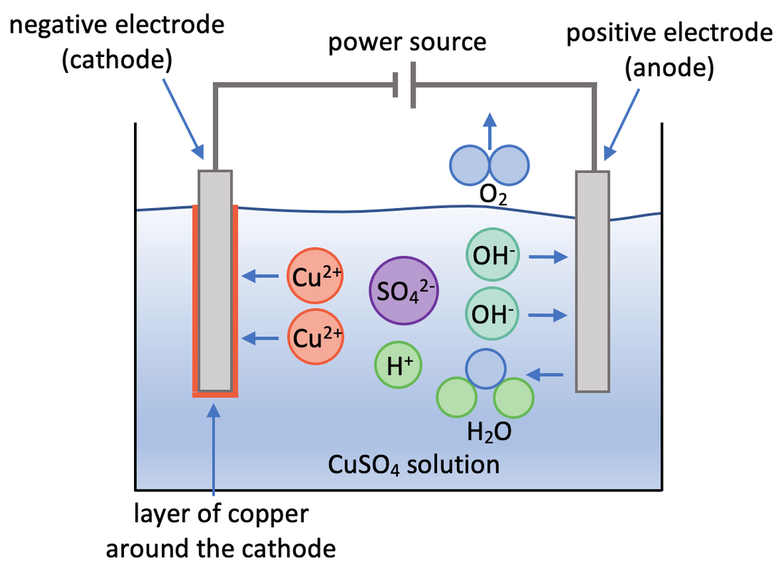
The first example that we are going to look at is the electrolysis of a copper (II) sulfate solution (CuSO4). There are 4 ions in this electrolyte; 2 from the ionic compound (Cu2+ and SO42-) and 2 from the water (H+ and OH-). The four ions are Cu2+, SO42-, H+ and OH-. A diagram for the electrolysis of copper (II) sulfate solution is shown below.
Copper is less reactive than hydrogen. This means that copper goes to the cathode (negative electrode), which results in a layer of copper forming around the cathode. At the cathode, each copper ion gains 2 electrons, which means that copper is reduced. The half equation for this is shown below.
There are no halide ions present. This means that the negative hydroxide ions go to the anode (positive electrode) where oxygen (O2) and water (H2O) are produced. The negative hydroxide ions lose electrons at the anode, which means that they are oxidised. The half equation for this is shown below.
The oxygen produced at the anode will bubble through the electrolyte and enter the air in the lab. We can test for the presence of oxygen by placing a glowing splint above where the oxygen is potentially produced; if oxygen is present, the glowing splint will reignite/ relight.
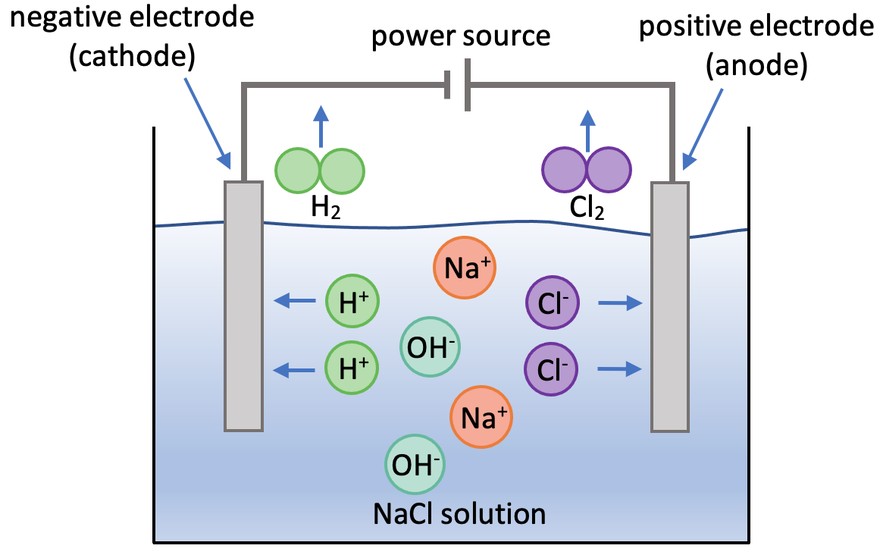
We are now going to look at the electrolysis of a sodium chloride solution (NaCl). There are 4 ions in this electrolyte; 2 from the ionic compound (Na+ and Cl-) and 2 from the water (H+ and OH-). The four ions are Na+, Cl-, H+ and OH-. A diagram for the electrolysis of sodium chloride solution is shown below.
Sodium is more reactive than hydrogen. This means that the hydrogen ions go to the cathode (negative electrode), which results in hydrogen gas being produced at the cathode (H2 gas is produced). At the cathode, each of the hydrogen ions gains 1 electron, which means that they are reduced. The half equation for this is shown below.
The hydrogen gas produced will bubble through the electrolyte. We can test for the presence of hydrogen by placing a lit splint above where the hydrogen is potentially produced; if hydrogen is present, we will hear a squeaky pop.
There are halide ions in the electrolyte – the halide ions present are Cl-. This means that the halide ions present will go to the anode (positive electrode) where molecules of the halogen will be produced; the halide ion in this electrolyte was chloride ions, so the chloride ions go to the anode where chlorine gas is produced (Cl2). The negative chloride ions lose electrons, which means that they are oxidised. The half equation for this is shown below.
The chlorine produced at the anode will bubble through the electrolyte. We can test for the presence of chlorine by holding damp blue litmus paper where the chlorine gas is produced; if chlorine is present, the chlorine will bleach the damp blue litmus paper turning it white (the litmus paper may turn red before it turns white because the solution of chlorine is acidic).