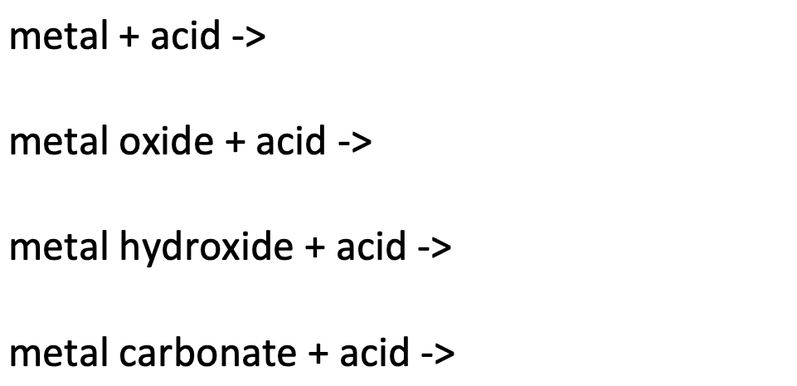Back to C4 Home
C4: Quiz 3
C4: Quiz 3
Click here for a printable PDF of question 1 & question 2 for this quiz.
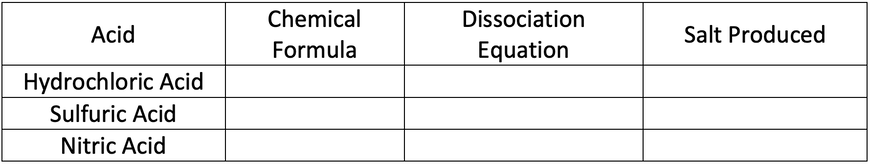
1) Complete the table below.
1) Complete the table below.
2) Write the products for the following reactions.
2) Write the products for the following reactions.
3)
a) What is the test for hydrogen?
b) What is the test for carbon dioxide?
4)
a) Write the word equation for the reaction of magnesium with hydrochloric acid.
b) Write the balanced chemical equation for this reaction.
5)
a) Write the word equation for the reaction of zinc with sulfuric acid.
b) Write the balanced chemical equation for this reaction.
6)
a) Write the word equation for the reaction of copper oxide with hydrochloric acid.
b) Write the balanced chemical equation for this reaction.
7)
a) Write the word equation for the reaction of potassium hydroxide with nitric acid.
b) Write the balanced chemical equation for this reaction.
8)
a) What is the chemical symbol for a carbonate?
b) What is the charge on a carbonate ion?
9)
a) Write the word equation for the reaction of calcium carbonate with hydrochloric acid.
b) Write the balanced chemical equation for this reaction.
10)
a) Write the word equation for the reaction of sodium carbonate with sulfuric acid.
b) Write the balanced chemical equation for this reaction.
11) This last question is very tricky.
a) Write the word equation for the reaction of aluminium with hydrochloric acid.
b)
i) When aluminium ionically bonds, what will the charge be on an aluminium ion?
ii) When chlorine ionically bonds, what will the charge be on a chloride ion?
iii) Hence or otherwise, what is the chemical formula for aluminium chloride?
c) Write the balanced chemical equation for this reaction.
3)
a) What is the test for hydrogen?
b) What is the test for carbon dioxide?
4)
a) Write the word equation for the reaction of magnesium with hydrochloric acid.
b) Write the balanced chemical equation for this reaction.
5)
a) Write the word equation for the reaction of zinc with sulfuric acid.
b) Write the balanced chemical equation for this reaction.
6)
a) Write the word equation for the reaction of copper oxide with hydrochloric acid.
b) Write the balanced chemical equation for this reaction.
7)
a) Write the word equation for the reaction of potassium hydroxide with nitric acid.
b) Write the balanced chemical equation for this reaction.
8)
a) What is the chemical symbol for a carbonate?
b) What is the charge on a carbonate ion?
9)
a) Write the word equation for the reaction of calcium carbonate with hydrochloric acid.
b) Write the balanced chemical equation for this reaction.
10)
a) Write the word equation for the reaction of sodium carbonate with sulfuric acid.
b) Write the balanced chemical equation for this reaction.
11) This last question is very tricky.
a) Write the word equation for the reaction of aluminium with hydrochloric acid.
b)
i) When aluminium ionically bonds, what will the charge be on an aluminium ion?
ii) When chlorine ionically bonds, what will the charge be on a chloride ion?
iii) Hence or otherwise, what is the chemical formula for aluminium chloride?
c) Write the balanced chemical equation for this reaction.