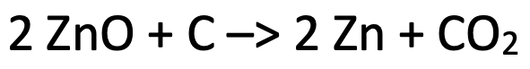Back to C4 Home
C4: Quiz 8
C4: Quiz 8
1) What is an ore?
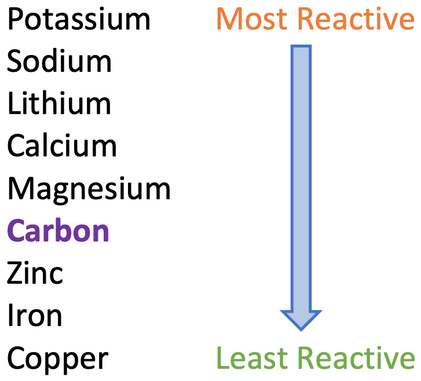
2) The reactivity series is given below.
2) The reactivity series is given below.
a) Give 2 ways that we can extract metals that are less reactive than carbon from their ores?
b) How can we extract metals that are more reactive than carbon from their ores?
c) Which is the more expensive process out of reduction with carbon and electrolysis?
3) Fill in the blanks below with the words “gain” and “loss”
With respect to oxygen, oxidation is the ______ of oxygen and reduction is the ______ of oxygen
4) The reaction between zinc oxide and carbon is shown below.
b) How can we extract metals that are more reactive than carbon from their ores?
c) Which is the more expensive process out of reduction with carbon and electrolysis?
3) Fill in the blanks below with the words “gain” and “loss”
With respect to oxygen, oxidation is the ______ of oxygen and reduction is the ______ of oxygen
4) The reaction between zinc oxide and carbon is shown below.
a) Which substance has been reduced? Explain your answer.
b) Which substance has been oxidised? Explain your answer.
5) Iron can be extracted from iron (III) oxide by using carbon to produce pure iron and carbon dioxide. Iron (III) oxide is Fe2O3.
a) Write the balanced chemical equation for this reaction.
b) Which substance has been reduced? Explain your answer.
c) Which substance has been oxidised? Explain your answer.
6) Copper can be extracted from copper oxide (CuO) to produce pure copper and carbon dioxide.
a) Write the balanced chemical equation for this reaction.
b) Which substance has been reduced? Explain your answer.
7) Why can’t calcium be extracted from calcium oxide by reduction using carbon?