Back to C6 Home
C6 F) Reversible Reactions
C6 F) Reversible Reactions
Some reactions are reversible. This means that the reaction can go in both directions; the reactants can react to form the products, and the products of a reaction can react to form the original reactants.
The double arrow in the above reaction equation tells us that the reaction is reversible – it can go in both directions. A and B can react together to produce C and D. And, C and D can react together to produce A and B.
The reactions in reversible reactions will go in both directions. If there are more reactions of A and B than the reactions of C and D, the quantity of A and B will increase as A and B are reacting, and the quantity of C and D will rise because A and B are reacting to produce C and D. As the concentration of A and B has fallen, there will be fewer reactions of A and B, and more reactions of C and D because the quantities of C and D have increased. Eventually the number of reactions of A and B will be the same as the number of reactions of C and D. This will mean that the quantities of all of the substances (A, B, C and D) in the reaction will remain the same. This point is known as a dynamic equilibrium. Reactions are still happening in a dynamic equilibrium, but the concentrations of the reactants and products remains constant because the same amount of A, B, C and D will be used in reactions as the amount that is produced from reactions; the amount of A and B that reacts to produce C and D, will be produced from the reaction of C and D to produce A and B. A dynamic equilibrium can only occur for a reversible reaction in a closed system, which is a system where none of the reactants or products are able to escape.
In a dynamic equilibrium, the concentrations of all of the substances remains constant. This does not mean that the concentration of all of the substances are equal. For example, a dynamic equilibrium for the made-up reaction at the top could be where 20% of the substances are A and B, and then 80% of the substances are C and D. Or, another dynamic equilibrium could be where 60% of the substances are A and B, and then 40% of the substances are C and D.
We can talk about the position of dynamic equilibriums by saying which side the equilibrium lies. If we say that the equilibrium lies to the right, it means that there is a greater concentration of the products (C and D) compared to the reactants (A and B). If we say that the equilibrium lies to the left, it means that there is a greater concentration of the reactants (A and B) compared to the products (C and D).
The reactions in reversible reactions will go in both directions. If there are more reactions of A and B than the reactions of C and D, the quantity of A and B will increase as A and B are reacting, and the quantity of C and D will rise because A and B are reacting to produce C and D. As the concentration of A and B has fallen, there will be fewer reactions of A and B, and more reactions of C and D because the quantities of C and D have increased. Eventually the number of reactions of A and B will be the same as the number of reactions of C and D. This will mean that the quantities of all of the substances (A, B, C and D) in the reaction will remain the same. This point is known as a dynamic equilibrium. Reactions are still happening in a dynamic equilibrium, but the concentrations of the reactants and products remains constant because the same amount of A, B, C and D will be used in reactions as the amount that is produced from reactions; the amount of A and B that reacts to produce C and D, will be produced from the reaction of C and D to produce A and B. A dynamic equilibrium can only occur for a reversible reaction in a closed system, which is a system where none of the reactants or products are able to escape.
In a dynamic equilibrium, the concentrations of all of the substances remains constant. This does not mean that the concentration of all of the substances are equal. For example, a dynamic equilibrium for the made-up reaction at the top could be where 20% of the substances are A and B, and then 80% of the substances are C and D. Or, another dynamic equilibrium could be where 60% of the substances are A and B, and then 40% of the substances are C and D.
We can talk about the position of dynamic equilibriums by saying which side the equilibrium lies. If we say that the equilibrium lies to the right, it means that there is a greater concentration of the products (C and D) compared to the reactants (A and B). If we say that the equilibrium lies to the left, it means that there is a greater concentration of the reactants (A and B) compared to the products (C and D).
Reversible Reaction Example 1
Here is an example of a real reversible reaction.
Here is an example of a real reversible reaction.
The above reaction can go in both directions. Ammonia chloride can react and breakdown to produce ammonia and hydrogen chloride. And, ammonia and hydrogen chloride can react to produce ammonia chloride. If this reversible reaction was taking place in a closed system, the reversible reaction would reach a dynamic equilibrium whereby the concentrations of all of the substances would remain constant.
Endothermic & Exothermic Reactions
One direction in a reversible reaction will be exothermic and the other direction will be endothermic. Exothermic reactions give out energy to their surroundings (this energy is usually given out in the form of heat). Endothermic reactions take in energy from their surroundings (the energy that is taken in is usually heat).
For the above reversible reaction of ammonia chloride, the forwards reaction is endothermic, and the backwards reaction is exothermic. The amount of energy that is taken in from the endothermic reaction will be the same as the amount of energy that is given out during the exothermic reaction.
One direction in a reversible reaction will be exothermic and the other direction will be endothermic. Exothermic reactions give out energy to their surroundings (this energy is usually given out in the form of heat). Endothermic reactions take in energy from their surroundings (the energy that is taken in is usually heat).
For the above reversible reaction of ammonia chloride, the forwards reaction is endothermic, and the backwards reaction is exothermic. The amount of energy that is taken in from the endothermic reaction will be the same as the amount of energy that is given out during the exothermic reaction.
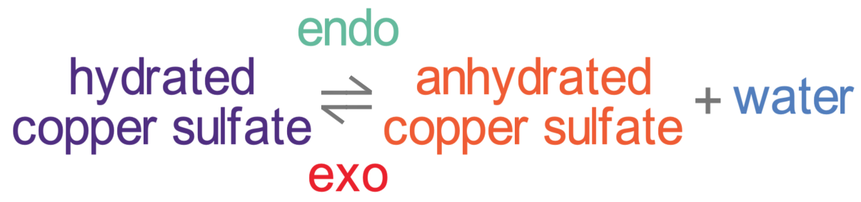
Hydrated Copper Sulfate
Another example of a reversible reaction is the thermal decomposition of hydrated copper sulfate.
Another example of a reversible reaction is the thermal decomposition of hydrated copper sulfate.
In the above equation hydrated means with water and anhydrous means without water.
The forwards reaction is endothermic and the backwards reaction is exothermic.
The forwards reaction is endothermic and the backwards reaction is exothermic.
- The hydrated copper sulfate crystals (II) are blue. If we heated them up, the water would leave resulting in white anhydrous copper (II) sulfate powder. We need to add heat to this reaction because the forwards reaction is endothermic, which means that it takes in energy from the surroundings.
- If we then added a few drops of water to the white anhydrous copper (II) sulfate powder, the backwards reaction would take place and blue crystals would appear again. The backwards reaction is exothermic.