Back to C6 Home
C6: Quiz 5 – Answers
C6: Quiz 5 – Answers
1) A catalyst is something that speeds up the rate of reaction and is not used up during the reaction/ remains unchanged during the reaction
2) Just certain reactions
3)
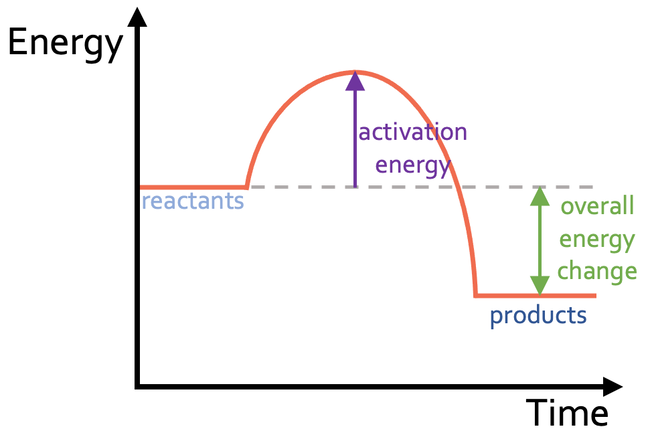
a) Activation energy is the amount of energy that is required to start a chemical reaction. It is the difference between the energy in the reactants and the highest energy point on the reaction profile
b) The overall energy change is the difference between the energy in the products and the energy in the reactants; overall energy change = energy in products – energy in reactants
4)
a) An exothermic reaction is a reaction that gives out energy (there is less energy in the products compared with the reactants)
b) You do not need to have labelled the activation energy and overall energy change
2) Just certain reactions
3)
a) Activation energy is the amount of energy that is required to start a chemical reaction. It is the difference between the energy in the reactants and the highest energy point on the reaction profile
b) The overall energy change is the difference between the energy in the products and the energy in the reactants; overall energy change = energy in products – energy in reactants
4)
a) An exothermic reaction is a reaction that gives out energy (there is less energy in the products compared with the reactants)
b) You do not need to have labelled the activation energy and overall energy change
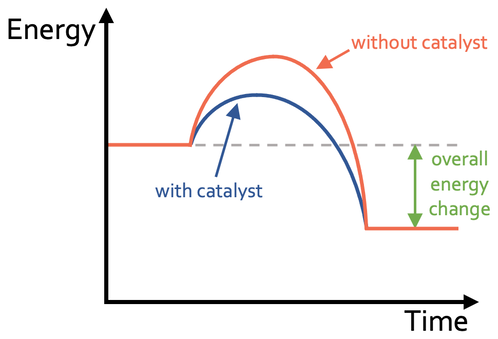
c) Reactants and products at the same level. Then a curve connecting the reactants and products with a lower activation energy.
d) Greater without a catalyst
e) No – the overall energy change remains the same
f) No – the chemical equation of the reaction stays the same
e) No – the overall energy change remains the same
f) No – the chemical equation of the reaction stays the same
Questions
1) What is a catalyst?
2) Will a catalyst catalyse all reactions or just certain reactions?
3)
a) Define activation energy.
b) Define overall energy change (OEC).
4)
a) What is an exothermic reaction?
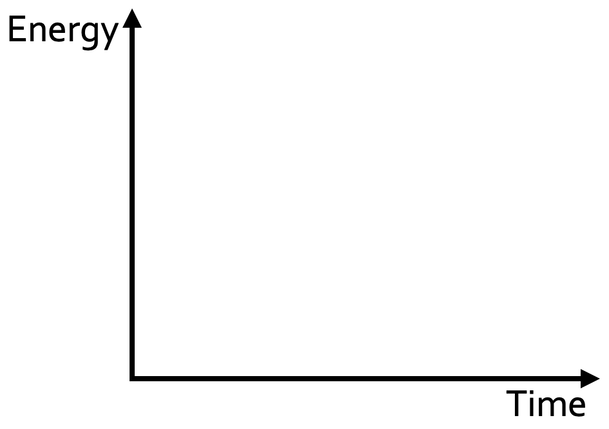
b) Draw the reaction profile for an exothermic reaction on the graph below.
1) What is a catalyst?
2) Will a catalyst catalyse all reactions or just certain reactions?
3)
a) Define activation energy.
b) Define overall energy change (OEC).
4)
a) What is an exothermic reaction?
b) Draw the reaction profile for an exothermic reaction on the graph below.
c) On the same graph that you have used to answer part b, draw the reaction profile for the same reaction when a catalyst is used.
d) Is the activation energy greater with a catalyst or greater without a catalyst?
e) Does the overall energy change (OEC) change when a reaction takes place with a catalyst?
f) Does the chemical equation for a reaction change when a catalyst is used to catalyse a reaction?
d) Is the activation energy greater with a catalyst or greater without a catalyst?
e) Does the overall energy change (OEC) change when a reaction takes place with a catalyst?
f) Does the chemical equation for a reaction change when a catalyst is used to catalyse a reaction?