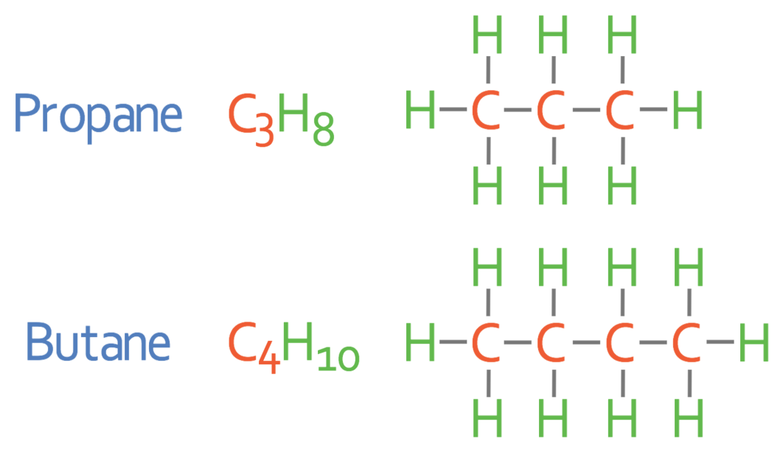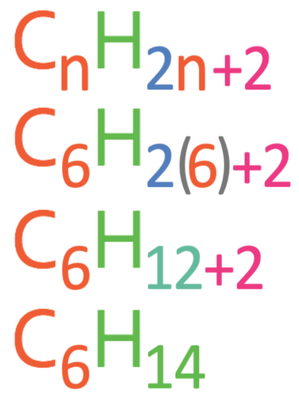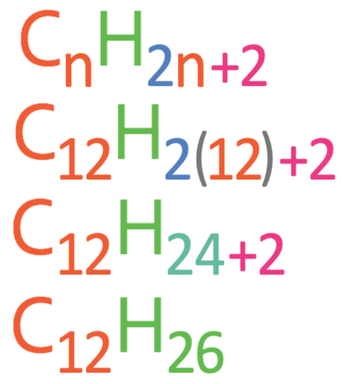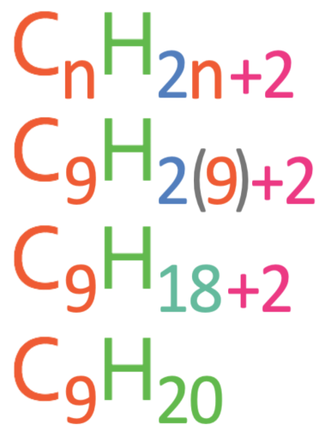C7 A) Hydrocarbons and Alkanes
Hydrocarbons are compounds that are made out of hydrogen and carbon atoms only (make sure that you include only in your definition). An example of a hydrocarbon is ethane, which has the formula C2H6. This is a hydrocarbon as it contains both hydrogen and carbon atoms only. Glucose has the chemical formula C6H12O6, and this is not a hydrocarbon because oxygen is present. So, a hydrocarbon is a compound that is made out of hydrogen and carbon atoms only.
Alkanes are made out of hydrogen and carbon only. Each of the carbon atoms forms four covalent bonds and each of the hydrogen atoms forms one covalent bond. A covalent bond is where two non-metals share pairs of electrons with each other; there is then a strong force of attraction between the positively charged nuclei of the atoms and the shared pair(s) of electrons. Alkanes are hydrocarbons where all of the carbon and hydrogen atoms have single bonds; they are known as being saturated hydrocarbons. Alkanes are different lengths because of the number of carbon atoms that are in them. Alkanes follow the general molecular formula:
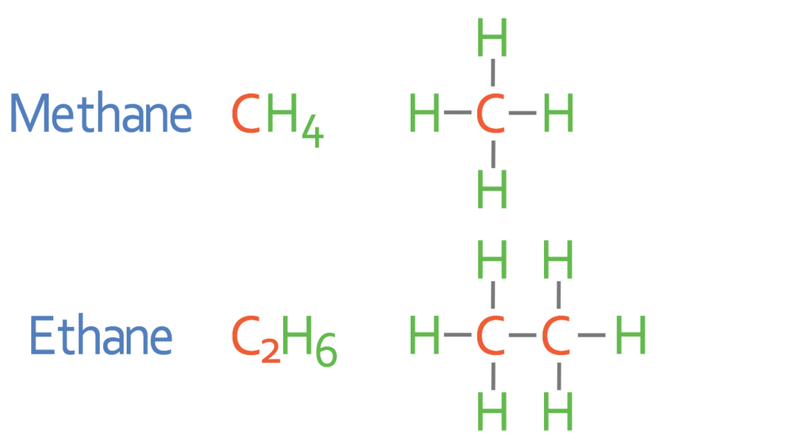
For the exam you need to know the first four alkanes. The names, molecular formulas and displayed formulas for the first four alkanes are shown below.
The molecular formula tells us the exact number and type of atoms in a single molecule of a compound. The molecular formula for propane is C3H8.
The diagrams next to the first four alkanes are known as the displayed formulas. The single “–” between each of the atoms indicates that there is a single covalent bond between the atoms.
Alkanes are a homologous series because they all react in a similar way.
Different hydrocarbons have different properties, such as different boiling points and different flammabilities:
- Longer hydrocarbons have higher melting and boiling points compared to shorter hydrocarbons. This is because longer hydrocarbons have stronger intermolecular forces of attraction, which require more energy to overcome and change their state, thus meaning that longer hydrocarbons have higher melting and boiling points (intermolecular forces of attraction are the forces of attraction between molecules). Shorter hydrocarbons tend to be gases at room temperature and longer hydrocarbons are liquids at room temperature. Shorter hydrocarbons are more volatile (volatility refers to how easy a substance will turn into a gas/ evaporate; shorter hydrocarbons have lower boiling points, so will turn into a gas more easily).
- Flammability refers to how easy it is to ignite (set fire to) the hydrocarbon. The shorter the hydrocarbon, the more flammable it is and the longer the hydrocarbon the less flammable it is.
- Viscosity refers to how easily a liquid (or gas) flows. A viscous liquid is quite gloopy (think golden syrup or tomato sauce) and a less viscous liquid will flow more (think water). The longer the hydrocarbon, the more vicious it is (like golden syrup). The shorter the hydrocarbon, the less viscous it is (like water).
The properties of the different hydrocarbons determines how the different hydrocarbons are used as fuels. We will be looking at the uses of the different hydrocarbons in more detail in the fractional distillation section.
The general molecular formula for alkanes is:
n in the formula above is the number of carbon atoms. We can use this formula to work out the molecular formula for an alkane with a certain number of carbon atoms.
Question: What is the molecular formula for an alkane with 6 carbons?
n in the general molecular formula is the number of carbon atoms. The question is asking us to find the molecular formula for an alkane with 6 carbons, which means that n in the formula is 6. We therefore sub n as 6 into the formula. The working is shown below.
This tells us that the molecular formula is C6H14.
Question: What is the molecular formula for an alkane with 12 carbons?
We find the molecular formula with 12 carbons by subbing n in as 12. The working is shown below.
The molecular formula is C12H26.
These questions can be made slightly harder by being asked to work out whether a hydrocarbon is or is not an alkane. The easiest way to answer these types of questions is to sub the number of carbons in the given hydrocarbon in as n into the general alkane formula (CnH2n+2) to find the number of hydrogens in the alkane with that number of carbons. If the number of hydrogens from the formula and the given hydrocarbon match, the given hydrocarbon is an alkane. Let’s have an example.
Question: Is the hydrocarbon C9H20 an alkane?
We answer this question by subbing the number of carbons in the given hydrocarbon in as n into the general alkane formula to find the number of hydrogens in the alkane with that number of carbons. The given hydrocarbon is C9H20, which has 9 carbons, so we sub n as 9 into the general alkane formula (CnH2n+2). The working is shown below.
According to the general alkane formula, the alkane with 9 carbons will have 20 hydrogens, which our given hydrocarbon does have. Therefore, C9H20 is an alkane.
For this example, the number of hydrogens matched. But, if the number of hydrogens did not match, the given hydrocarbon would not be an alkane.