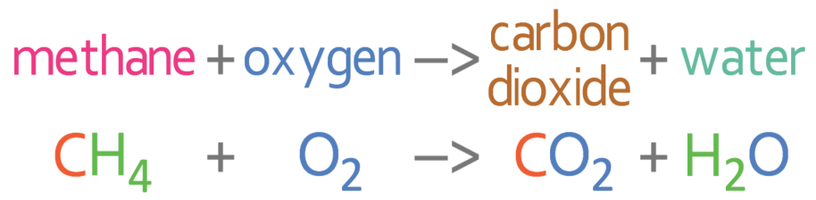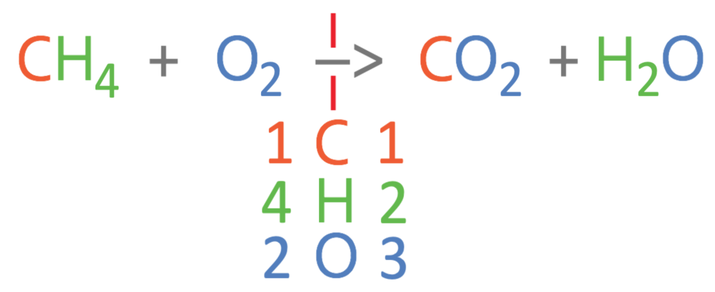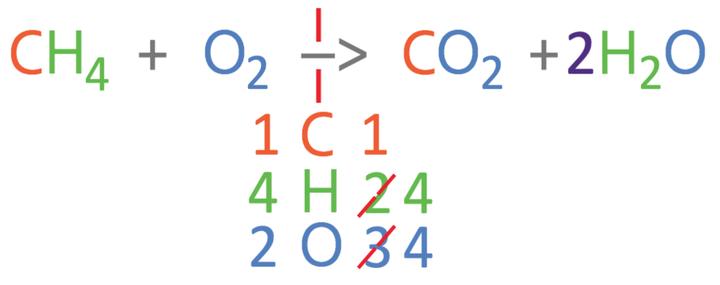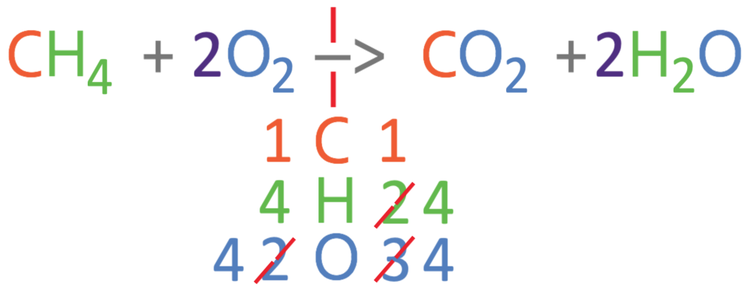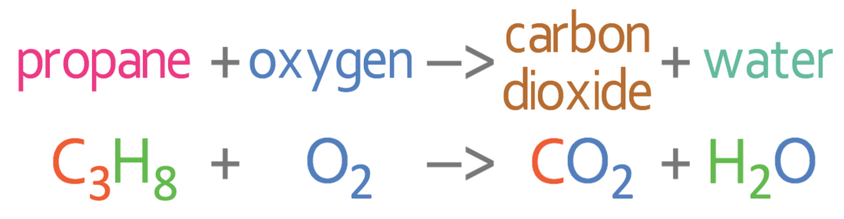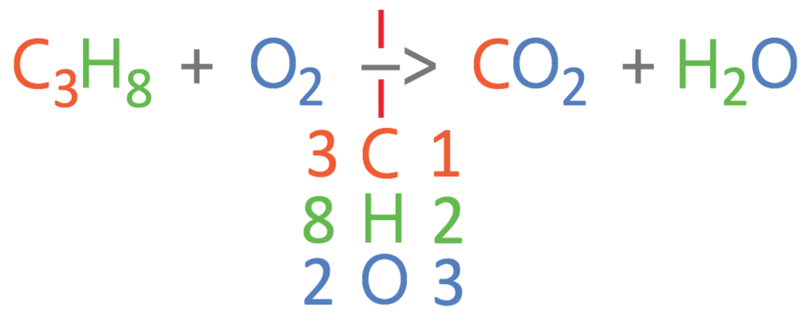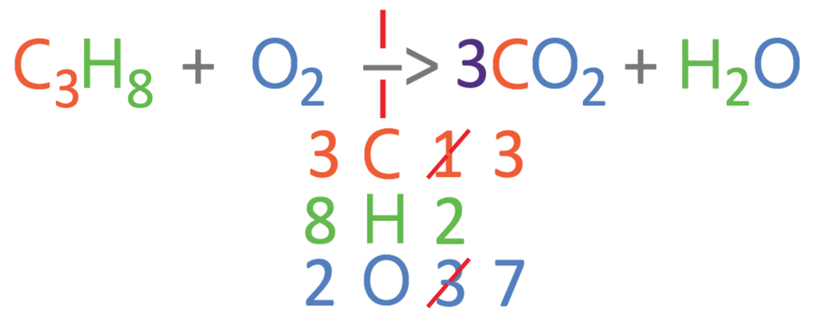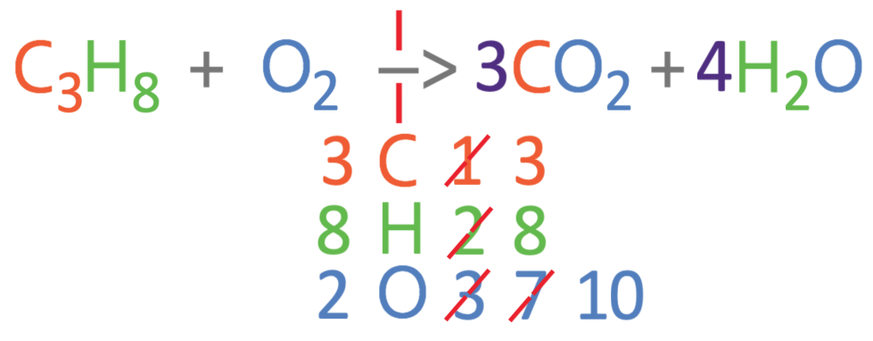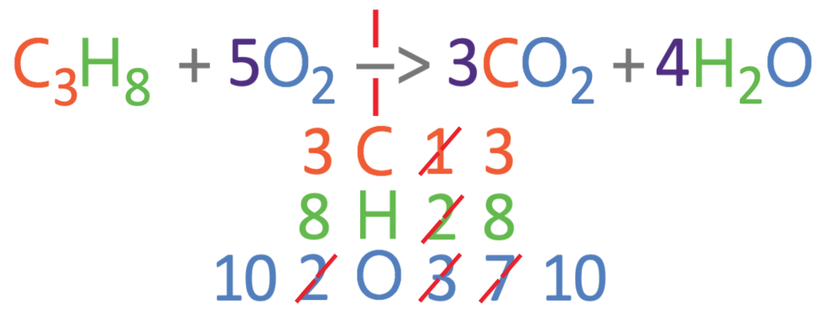C7 B) Combustion
The combustion of hydrocarbons releases a large quantity of energy, which can be used for a variety of different activities. For example, some gas BBQs burn butane (C4H10) to produce heat, which cooks the food. Another example would be the combustion of octane (C8H18) in a car’s engine to produce the energy needed to cause the car to move. As energy is released during a combustion reaction, it means that the combustion reaction is an exothermic reaction (energy is given out).
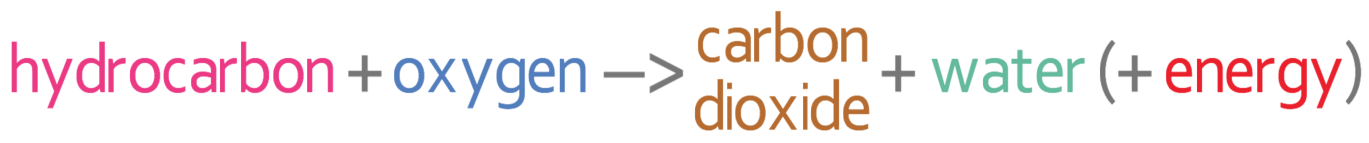
Complete combustion of a hydrocarbon happens when there is plenty of oxygen available. The combustion reaction produces carbon dioxide, water and energy. The word equation for complete combustion is:
Incomplete combustion occurs when there is not enough oxygen available for the reaction. The products of incomplete combustion are water (same as complete combustion), carbon monoxide and carbon (carbon monoxide and carbon are instead of carbon dioxide in complete combustion. The carbon that is produced during incomplete combustion is sometimes referred to as soot). Incomplete combustion releases energy, but it releases less energy than complete combustion. We will be looking at incomplete combustion in more detail in the pollution section.
We are now going to look at balancing the symbol equation for the complete combustion of the hydrocarbon methane (CH4).
The complete combustion reaction is the reaction of a hydrocarbon (for this example methane: CH4) and oxygen, to give us carbon dioxide and water. The unbalanced equation is shown below:
The first step in balancing the equation is to work out how many of the different elements we have on each side of the reaction. There are 3 different elements in this reaction; carbon, hydrogen and oxygen. I am going to place these 3 elements underneath the reaction arrow and then work out the number of the 3 different elements in the reactants (left) and the products (right). For the left side of the reaction, there is 1 carbon atom, 4 hydrogen atoms and 2 oxygen atoms. For the right side of the reaction, there is 1 carbon atoms, 2 hydrogen atoms and 3 oxygen atoms (2 from the CO2 and 1 from the H2O).
From comparing the numbers on the left and right, we can see that the carbons are balanced, but the hydrogens and oxygens are not balanced. I am going to balance the hydrogens first; currently there are 4 hydrogens on the left and 2 hydrogens on the right. We can make the hydrogens balance by having 2 waters on the right, which will give us 4 hydrogen atoms on the right. As we are having 2 waters on the right, we place a big 2 in front of the water (2H2O). As we have made a modification to the right side of the reaction, we should count the number of all 3 elements again (it is always worth counting again to avoid making a mistake). On the right, there is 1 carbon atom, 4 hydrogen atoms and 4 oxygen atoms (2 from the CO2 and 2 from the 2H2O). The updated equation is shown below.
The carbons and hydrogens are balanced, but the oxygens are not balanced. Currently, there are 2 oxygens on the left and 4 oxygens on the right. We are able to balance the oxygens by placing a 2 in front of the oxygen (2O2). We have made a modification to the left side of the reaction, so I am going to count the number of each of the 3 different elements again; on the left side of the equation, we have 1 carbon atom, 4 hydrogen atoms and 4 oxygen atoms.
The numbers of the 3 different elements are balanced; we have 1 carbon atom on both sides, 4 hydrogen atoms on both sides and 4 oxygen atoms on both sides. We now have the balanced equation.
Whenever we are balancing complete combustion equations, it is best to balance the carbons and hydrogens before balancing the oxygens.
Write the balanced symbol equation for the complete combustion of propane (C3H8).
Feel free to give this question a go by yourself!
The complete combustion reaction is the reaction of a hydrocarbon (for this example propane: C3H8) and oxygen, to give us carbon dioxide and water. The unbalanced equation is shown below:
The first step in balancing the equation is to work out how many carbons, hydrogens and oxygens there are on the left and right side of the equation. For the left side of the equation, there are 3 carbons, 8 hydrogens and 2 oxygens. On the right side of the equation, there is 1 carbon, 2 hydrogens and 3 oxygens (2 from the CO2 and 1 from the H2O).
From comparing the numbers on the left and right, we can see that all 3 of the different elements are not balanced. I am going to balance the carbons first. Currently there are 3 carbons on the left and 1 carbon on the right. We can make the carbons balance by having 3 carbon dioxides on the right side of the reaction; we add a big 3 in front of the carbon dioxide (3CO2). As we have made a modification to the right side of the reaction, we should count the number of all 3 elements again. On the right, there are 3 carbon atoms, 2 hydrogen atoms and 7 oxygen atoms (6 from the 3CO2 and 1 from the H2O). The updated equation is shown below.
The carbons are now balanced, but the hydrogens and oxygens are not balanced. I am going to balance the hydrogens next. Currently, there are 8 hydrogens on the left and 2 on the right. We are able to make the hydrogens balance by having 4 waters on the right side of the reaction; we place a big 4 in front of the water (4H2O). We now need to count the number of the 3 different elements on the right side of the reaction. On the right, there are 3 carbon atoms, 8 hydrogen atoms and 10 oxygen atoms (6 from the 3CO2 and 4 from the 4H2O). The updated equation is shown below.
The carbons and hydrogens are balanced, but the oxygens are not balanced. Currently, there are 2 oxygens on the left and 10 oxygens on the right. We are able to balance the oxygens by having 5 oxygens on the left side of the reaction; we place a big 5 in front of the oxygens (5O2). We now need to count all of the 3 different elements on the left side of the reaction. On the left, there are 3 carbons, 8 hydrogens and 10 oxygens.
The numbers of the 3 different elements all balance now; we have 3 carbon atoms on both sides, 8 hydrogen atoms on both sides and 10 oxygen atoms on both sides. We now have the balanced equation.
The balancing of reactions isn’t too tricky providing that you are careful and methodical in your working.