Back to C7 Home
C7 C) Fractional Distillation
C7 C) Fractional Distillation
Crude Oil
Crude oil is a fossil fuel. It was formed from dead plants and animals (mainly plankton) that were buried in mud millions of years ago. Over millions of years, a high temperature and pressure has caused the remains of the dead plants and animals to turn into crude oil. We can extract crude oil from the ground and use it for a variety of different uses.
Crude oil is used in transportation (cars, trains, boats etc.), in the house (in a boiler and gas cooking hobs) and is used to create plastics and chemicals (such as polymers, lubricants, detergents and solvents).
Crude oil is a non-renewable resource meaning that it will run out one day; it is known as being finite. Energy companies are constantly looking for new oil reserves and are advancing technologies so that crude oil can be extracted from parts of the world where we previously wouldn’t have been able to extract crude oil from (such as tar sands).
Crude oil is a fossil fuel. It was formed from dead plants and animals (mainly plankton) that were buried in mud millions of years ago. Over millions of years, a high temperature and pressure has caused the remains of the dead plants and animals to turn into crude oil. We can extract crude oil from the ground and use it for a variety of different uses.
Crude oil is used in transportation (cars, trains, boats etc.), in the house (in a boiler and gas cooking hobs) and is used to create plastics and chemicals (such as polymers, lubricants, detergents and solvents).
Crude oil is a non-renewable resource meaning that it will run out one day; it is known as being finite. Energy companies are constantly looking for new oil reserves and are advancing technologies so that crude oil can be extracted from parts of the world where we previously wouldn’t have been able to extract crude oil from (such as tar sands).
Fractional Distillation
Crude oil is a mixture of lots of different hydrocarbons (hydrocarbons are compounds made out of hydrogen and carbon only). Each of the different hydrocarbons in crude oil have different boiling points. The longer hydrocarbons have higher boiling points, and the shorter hydrocarbons have lower boiling points.
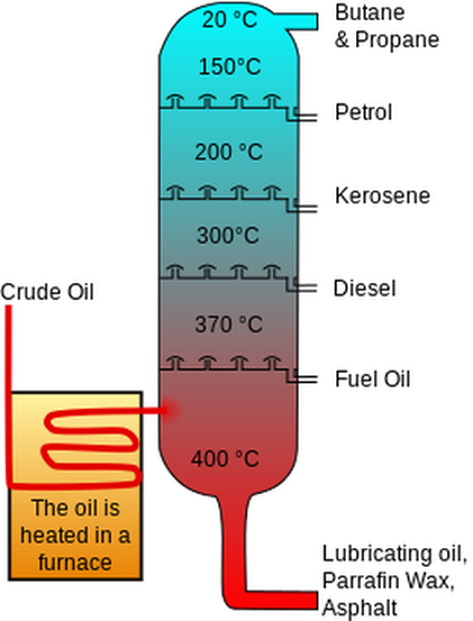
We can separate the different hydrocarbons in crude oil by using fractional distillation. A diagram for fractional distillation is shown below:
Crude oil is a mixture of lots of different hydrocarbons (hydrocarbons are compounds made out of hydrogen and carbon only). Each of the different hydrocarbons in crude oil have different boiling points. The longer hydrocarbons have higher boiling points, and the shorter hydrocarbons have lower boiling points.
We can separate the different hydrocarbons in crude oil by using fractional distillation. A diagram for fractional distillation is shown below:
In fractional distillation, crude oil is heated up to around 350°C before it is pumped into the column. The column is the hottest at the bottom and the coolest at the top. Most of the hydrocarbons in the heated crude oil will be gases, which means that they rise up the column. The really long hydrocarbons (such a bitumen) have a higher boiling point than the temperature that they are heated up to, which means that they remain as a liquid at the bottom of the column.
The hydrocarbons that are gases rise up the column until they reach a level in the column where the temperature is their boiling point. At this level/ temperature, they condense (turn into a liquid) and are then drained out of the column. For the longer hydrocarbons, this happens at a lower level in the column, and for the shorter hydrocarbons, this happens at a higher level in the column. For example, the first level has a temperature of around 340°C and at this temperature, heavy fuel oil turns into a liquid (heavy fuel oil has around 40 carbon atoms). At 340°C all of the other shorter hydrocarbons are still gases, so they continue to rise up the column. The next level is at a temperature of 250°C and at this temperature, diesel turns into a liquid and all of the other shorter hydrocarbons rise further up (diesel has around 20 carbon atoms and is used as fuel for lorries). This process keeps continuing. The next hydrocarbon to condense in the column is kerosene, which has around 15 carbon atoms and is used as jet fuel. The next hydrocarbon to condense is petrol, which has around 8 carbon atoms and is used as fuel for cars. The final hydrocarbon is LPG (liquefied petroleum gas – mainly propane and butane), which is used in BBQs and gas heaters.
No reactions take place during fractional distillation. Instead, fractional distillation is a process that we use to separate crude oil (a mixture of hydrocarbons) to obtain the different hydrocarbons on their own; fractional distillation is a separation technique.
The hydrocarbons that are gases rise up the column until they reach a level in the column where the temperature is their boiling point. At this level/ temperature, they condense (turn into a liquid) and are then drained out of the column. For the longer hydrocarbons, this happens at a lower level in the column, and for the shorter hydrocarbons, this happens at a higher level in the column. For example, the first level has a temperature of around 340°C and at this temperature, heavy fuel oil turns into a liquid (heavy fuel oil has around 40 carbon atoms). At 340°C all of the other shorter hydrocarbons are still gases, so they continue to rise up the column. The next level is at a temperature of 250°C and at this temperature, diesel turns into a liquid and all of the other shorter hydrocarbons rise further up (diesel has around 20 carbon atoms and is used as fuel for lorries). This process keeps continuing. The next hydrocarbon to condense in the column is kerosene, which has around 15 carbon atoms and is used as jet fuel. The next hydrocarbon to condense is petrol, which has around 8 carbon atoms and is used as fuel for cars. The final hydrocarbon is LPG (liquefied petroleum gas – mainly propane and butane), which is used in BBQs and gas heaters.
No reactions take place during fractional distillation. Instead, fractional distillation is a process that we use to separate crude oil (a mixture of hydrocarbons) to obtain the different hydrocarbons on their own; fractional distillation is a separation technique.