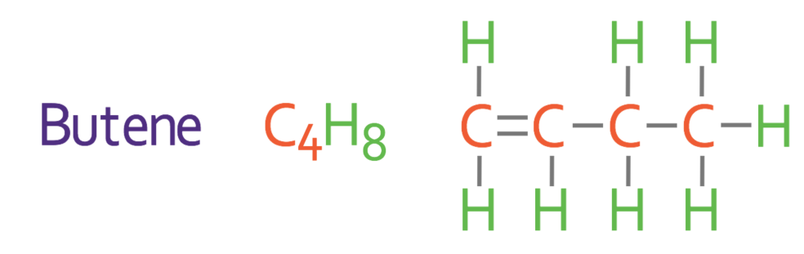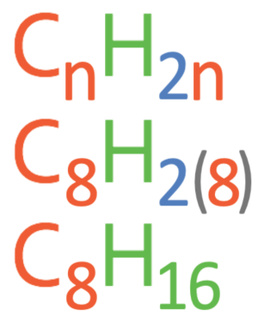C7 D) Alkenes
Alkanes and alkenes are hydrocarbons, which are molecules that contain hydrogen and carbon only. Alkanes are sometimes referred to as saturated hydrocarbons because all of the covalent bonds in the alkanes are single bonds; we cannot fit any more hydrogens on alkanes. Alkanes have the general molecular formula CnH2n+2.
Alkenes are hydrocarbons that contain a double carbon bond. Alkenes are sometimes referred to as unsaturated hydrocarbons because of the presence of the double carbon bond; if we got rid of the double carbon bond, we could fit more hydrogens onto the molecule, hence why alkenes are sometimes called unsaturated hydrocarbons. The double bond is represented with a double line “=”; it looks like an equals sign (C=C). Alkenes have the general formula:
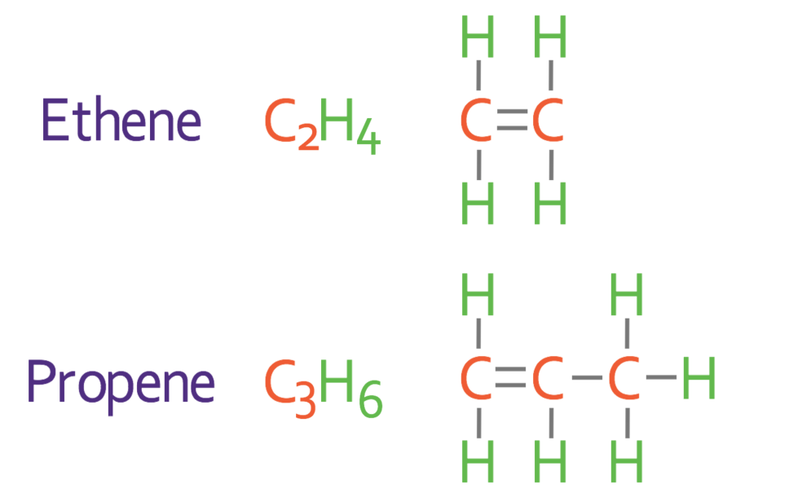
The names, molecular formulas and displayed formulas for the first three alkenes are shown below.
We can use the number of e’s in alkane and alkene to remember the type of bonds that the hydrocarbon has. Alkane has one e, so it has single bonds. Alkene has two e’s so it has a double bond. Also, we can tell whether a hydrocarbon is an alkane or an alkene by looking at the ending of the hydrocarbon’s name. All alkanes have the ending “ane”, and all alkenes have the ending “ene”. For example, propane (C3H8) is an alkane (it has the ending “ane”), and propene (C3H6) is an alkene (it has the ending “ene”).
Alkenes are considerably more reactive than alkanes. Also, they are used as a starting material for making other compounds, such as polymers.
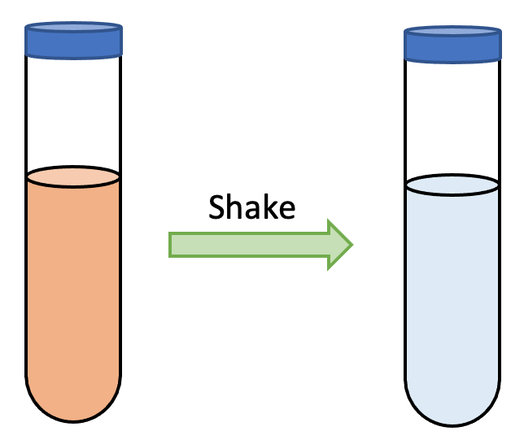
We can test whether a hydrocarbon is an alkane or an alkene by using bromine water. Bromine water is orange. We add a few drops of our hydrocarbon that we are testing to the bromine water and shake the test tube. These are the outcomes:
- If the hydrocarbon is an alkane, no reaction will take place and the solution in the test tube will remain orange.
- However, if the hydrocarbon is an alkene, the bromine will react with the alkene, which results in the solution going from orange to colourless. The change in colour of the solution occurs because the bromine reacts with the double carbon bond in the alkene.
The general molecular formula for alkenes is:
n in the formula above is the number of carbon atoms. We can use this formula to work out the molecular formula for an alkene with a certain number of carbon atoms.
Questions: What is the molecular formula for an alkene with 8 carbons?
n in the general formula is the number of carbon atoms. The question is asking us to find the molecular formula for an alkene with 8 carbons, which means that n in the formula is 8. We therefore sub n as 8 into the formula. The working is shown below.
This tells us that the molecular formula is C8H16.
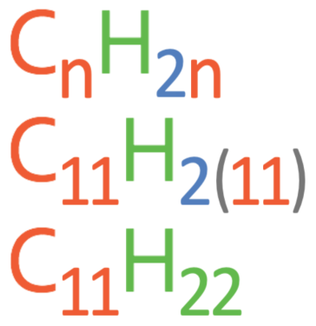
Question: What is the molecular formula for an alkene with 11 carbons?
We find the molecular formula with 11 carbons by subbing n in as 11. The working is shown below.
The molecular formula is C11H22.
These questions can be made slightly harder by being asked to work out whether a hydrocarbon is or is not an alkene. The easiest way to answer these types of questions is to sub the number of carbons in the given hydrocarbon in as n into the general alkene formula (CnH2n) to find the number of hydrogens in the alkene with that number of carbons. If the number of hydrogens from the formula and the given hydrocarbon match, the given hydrocarbon is an alkene. Let’s have an example.
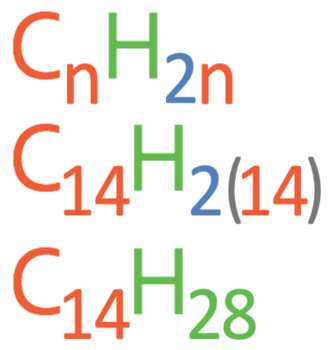
Question: Is the hydrocarbon C14H30 an alkene?
We answer this question by subbing the number of carbons in the given hydrocarbon in as n into the general alkene formula to find the number of hydrogens in the alkene with that number of carbons. The given hydrocarbon is C14H30, which has 14 carbons, so we sub n as 14 into the general alkene formula (CnH2n). The working is shown below.
According to the general alkene formula, the alkene with 14 carbons will have 28 hydrogens, which our given hydrocarbon does not have (our given hydrocarbon has 30 hydrogens). Therefore, C14H30 is not an alkene.