C7 E) Cracking
Cracking is a thermal decomposition reaction, which is the breaking down of molecules using heat (thermal decomposition is an endothermic reaction as energy/ heat is taken in). There are 2 different types of cracking; catalytic cracking and steam cracking.
The steps for catalytic cracking are:
- Heat up the long-chained hydrocarbon so that they turn into a gas (vaporise)
- Pass the vaporised long-chained hydrocarbon over a powdered catalyst sometimes aluminium oxide (a catalyst is something that increases the rate of reaction without being used up in the reaction)
- Thermal decomposition occurs as the longer chained hydrocarbon splits up into smaller chained hydrocarbons. One of the hydrocarbons produced will be an alkane and the other will be an alkene.
The process for steam cracking is essentially the same. The only difference is in step 2 where the vaporised long-chained hydrocarbon is mixed with steam rather than a powdered catalyst.
The cracking of a long-chained alkane will produce a shorter alkane and an alkene. There are many different combinations of cracked outcomes that can be produced. When we are writing cracking equations, we need to make sure that they balance; we have the same number of each of the elements on both sides of the reaction arrow.
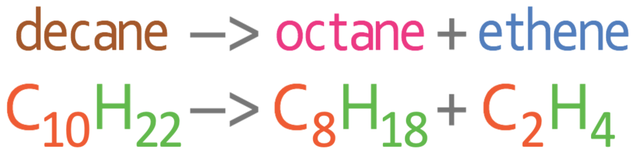
We are now going to write an equation for the cracking of decane (C10H22) to produce octane (C8H18) and the alkene ethene (C2H4). The word equation and balanced symbol equation for this cracking reaction is shown below.
With all chemical reactions, we need to make sure that we have the same number of each of the different elements on the left and right side of the reaction. Let’s check that this is the case for the above reaction. On the left side of the reaction there are 10 carbons and 22 hydrogens. On the right side of the reaction, there are 10 carbons (8 from C8H18 and 2 from C2H4) and 22 hydrogens (18 from C8H18 and 4 from C2H4). The numbers for each of the elements on both sides of the reaction balance, which means that the reaction is balanced.
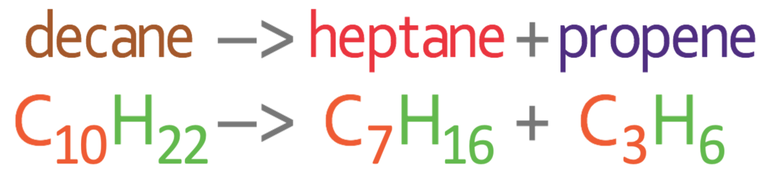
Like I mentioned earlier, there could be many different combinations of shorter alkanes and alkenes. For example, it may be the case that the decane (C10H22) could crack to produce heptane (C7H16) and propene (C3H6).
The reason why cracking is useful is because it matches supply and demand for the different hydrocarbons. For example, there is more decane in crude oil than the amount of decane that is demanded by users/ consumers. Therefore, we can crack decane to create octane and ethene. The octane is petrol, so is used in engines for small vehicles (cars and motorbikes). The ethene can be made into polythene, which is a type of plastic [polythene is also known as poly(ethene)].
Sometimes it can be the case that we crack a longer-chained alkane to produce a shorter alkane and many alkene molecules. We answer questions like this by balancing the number of carbons and hydrogens in the cracking equation; when we balance, we focus on the carbons. Let’s have a look at an example.
Question: The alkane C18H38 can be cracked to produce octane (C8H18) and many ethenes (C2H4). The partially completed cracking equation is shown below.
Balance the above cracking equation.
The only gap that we are given in the equation is the gap in front of ethene (C2H4); therefore, we can only add a big number in front of ethene. When we balance cracking equations, we need to make sure that we have the same number of hydrogens and carbons on both sides of the reaction. It is easier to balance cracking equations by focusing on the carbons.
There are 18 carbons on the left side of the equation.
On the right side of the equation, there are 8 carbons from the octane (C8H18). This means that there needs to be 10 more carbons coming from the ethenes on the right side of the equation to ensure that there are 18 carbons in total. In each ethene molecule there are 2 carbons. Therefore, in order to have 10 carbons coming from the ethenes, we need to have 5 ethene molecules; we place a big 5 in front of the ethene. This is shown below.
We can check that we have put in the correct number by counting the hydrogens. On the left side of the equation there are 38 hydrogens coming from the C18H38. On the right side of the equation there is also 38 hydrogens (18 from the C8H18 and 20 from the 5 C2H4). The hydrogens and carbons are balanced, so we have successfully balanced the cracking equation.