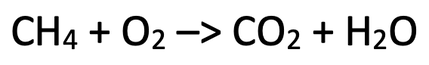C7: Quiz 2
1) Write down the general word equation for the complete combustion of a hydrocarbon.
2)
a) When does incomplete combustion occur?
b) What are the 3 potential products of incomplete combustion? (Do not say energy)
c) Out of complete combustion and incomplete combustion, which releases more energy?
3) The equation below shows the complete combustion of methane. Balance this chemical equation.
4) Write the balanced equation for the complete combustion of pentane (C5H12).
5)
a) An alkane contains 7 carbon atoms. Write the formula for this alkane.
b) Write the balanced chemical equation for the complete combustion of this hydrocarbon.
6) Write the balanced chemical equation for the complete combustion of the alkane C10H22.