C8: Quiz 2
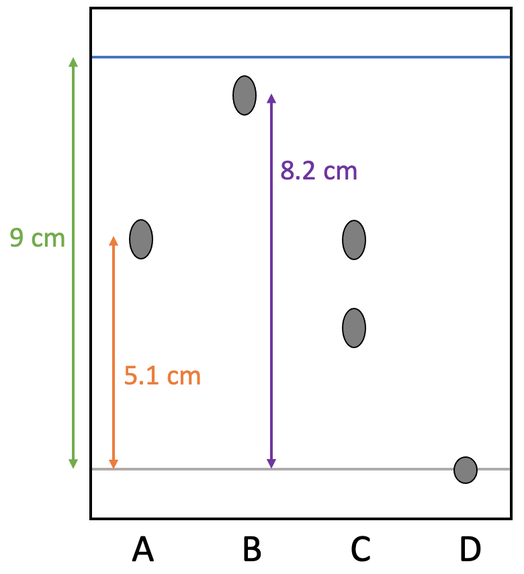
1) Paper chromatography was carried out by a student on the four dyes A, B, C and D. The solvent used was water. The chromatogram is show below.
a) We use pencil to draw the baseline. Why do we use pencil and not ink?
b) Dye D has stayed on the baseline. What does this tell us about dye D?
c) Dyes A, B and D have one spot. What does this imply about the compositions of these dyes?
d) Dye C has two spots. What does this tell us about the composition of dye C?
e) What is the name of the line at the top of the chromatogram?
f) Calculate the Rf value for dye A. Give your answer to 2 decimal places.
g) Calculate the Rf value for dye B. Give your answer to 2 decimal places.
h) The student now carries out the chromatography process again, but this time uses ethanol rather than water. Will the Rf values for all of the substances stay the same? Explain your answer.
2)
a) What are the two different phases in chromatography?
b) With respect to these two phases, why do the substances move different distances on the chromatogram?
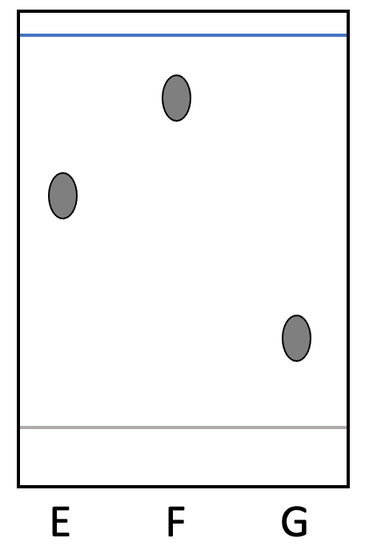
c) We have the chromatogram below for dyes E, F and G. All of these dyes are pure substances.
i) Which of these dyes spends the longest time in the mobile phase compared to the stationary phase? Explain your answer.
ii) Which of these dyes spends the longest time in the stationary phase compared to the mobile phase? Explain your answer.
3) A student carried out chromatography and used water as the solvent. He worked out the Rf value for a particular substance to be 0.46.
The student now carries out the chromatography process again, but this time uses ethanol rather than water. The particular substance is more soluble in ethanol compared to water. What will happen to the Rf value; will it increase or decrease? Explain your answer.
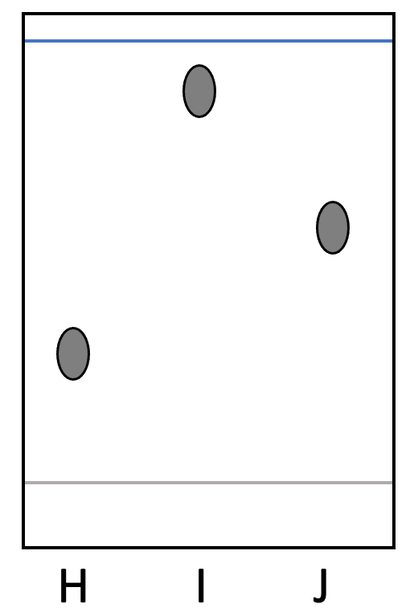
4) A student completes a chromatography experiment of 3 different pure substances; H, I and J. His chromatogram is show below.
a) Find the Rf values for H, I and J. Give your answers to 2 decimal places.
Note: you can answer this question by measuring the distances on your computer, tablet or phone – you do not need a printed version.
b) How could the student modify the chromatography experiment to get a more accurate Rf value?
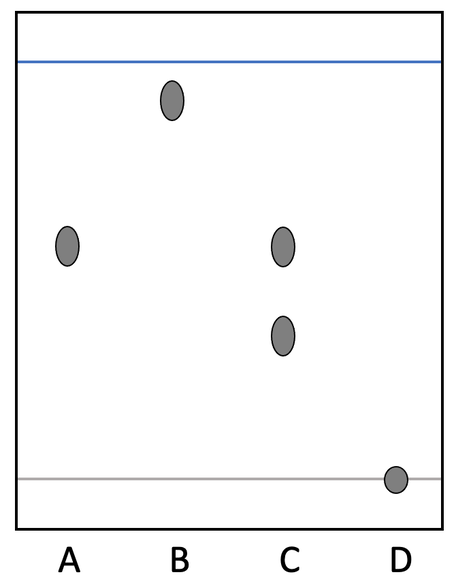
5) A student carries out a chromatography experiment and one of the samples stayed on the baseline; sample D stayed on the baseline. The chromatogram is shown below.
Explain a change that the student could make to the chromatography experiment to separate the substances in sample D.
6) How can we use chromatography to prove that a certain substance is present in a mixture?