C4: Quiz 10 – Answers
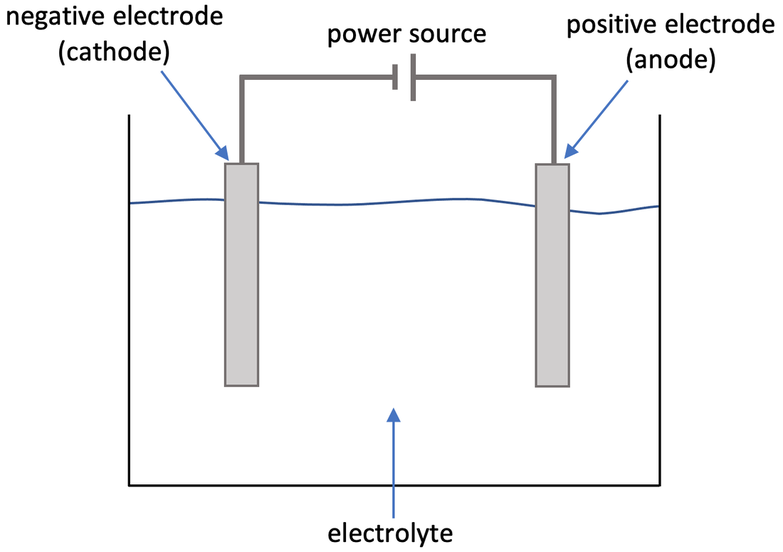
a) Electrolysis is the splitting up of an ionic compound by using electricity
b)
c) It is a relatively expensive process because vast quantities of electricity are required
2)
a) The ions in a solid ionic compound are unable to move. This means that solid ionic compounds cannot be split up using electricity
b) The electrolyte must be molten (liquid) or dissolved in water (aqueous)/ dissolved in another substance. This is so that the ions can move
3)
a) Anode
b) Cathode
4) Oxidation Is Loss, Reduction is Gain
5)
a) Al3+ and O2-
b)
i) Cathode (negative electrode)
ii) Al3+ + 3 e- –> Al
iii) Reduced as it has gained electrons
c)
i) Anode (positive electrode)
ii) 2 O2- –> O2 + 4 e-
iii) Oxidised as it has lost electrons
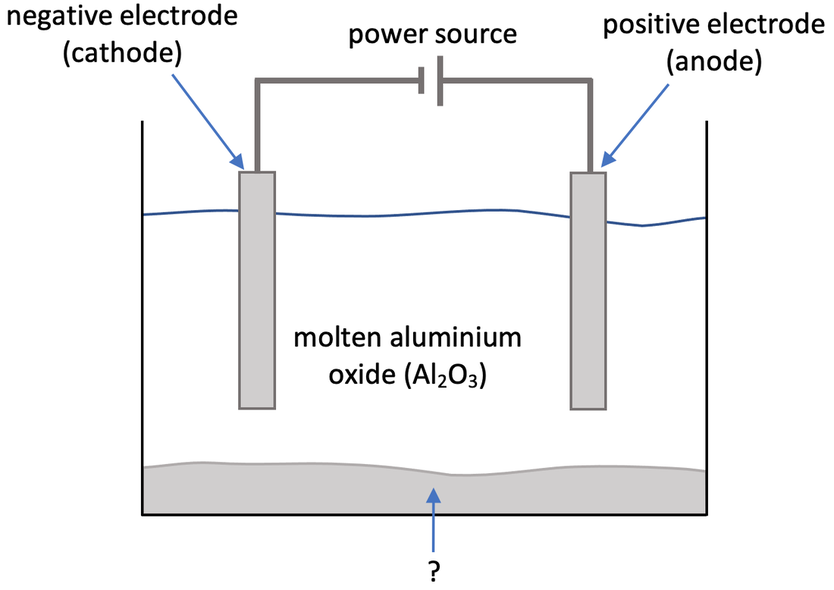
d) We dissolve powdered aluminium oxide in molten cryolite to lower the melting point, which requires less energy, thus reducing the cost of the electrolysis process
e) Aluminium or molten aluminium
6)
a) Pb2+ and Br-
b)
i) Pb2+ + 2 e- –> Pb
ii) Reduced as it has gained electrons
c)
i) 2 Br- –> Br2 + 2 e-
ii) Oxidised as it has lost electrons
7)
a) K+ and Cl-
b) 2 Cl- –> Cl2 + 2 e-
c) K+ + e- –> K
1)
a) What is electrolysis?
b) Draw the set up for electrolysis.
c) Is electrolysis a relatively cheap or expensive process? Explain your answer.
2)
a) Why can’t the electrolyte for electrolysis be a solid form of the ionic compound?
b) What state/ condition must the electrolyte be? Explain your answer.
3) There are two electrodes in electrolysis.
a) What is the name for the positive electrode?
b) What is the name for the negative electrode?
4) What does OIL RIG stand for?
5) This question is all about obtaining aluminium from aluminium oxide (Al2O3). The step-up of the electrolysis experiment is shown below.
a) What are the charges on the aluminium and oxide ions?
b)
i) Which electrode are the aluminium ions attracted to?
ii) Write the half equation for aluminium ions.
iii) With respect to electrons, has aluminium been oxidised or reduced? Explain your answer.
c)
i) Which electrode are the oxide ions attracted to?
ii) Write the half equation for oxide ions.
iii) With respect to electrons, has oxygen been oxidised or reduced? Explain your answer.
d) Instead of having the electrolyte as molten aluminium oxide, we can dissolve powdered aluminium oxide into molten cryolite. Why is it beneficial to do this?
e) What is the name of the substance labelled with a question mark on the diagram at the start of the question?
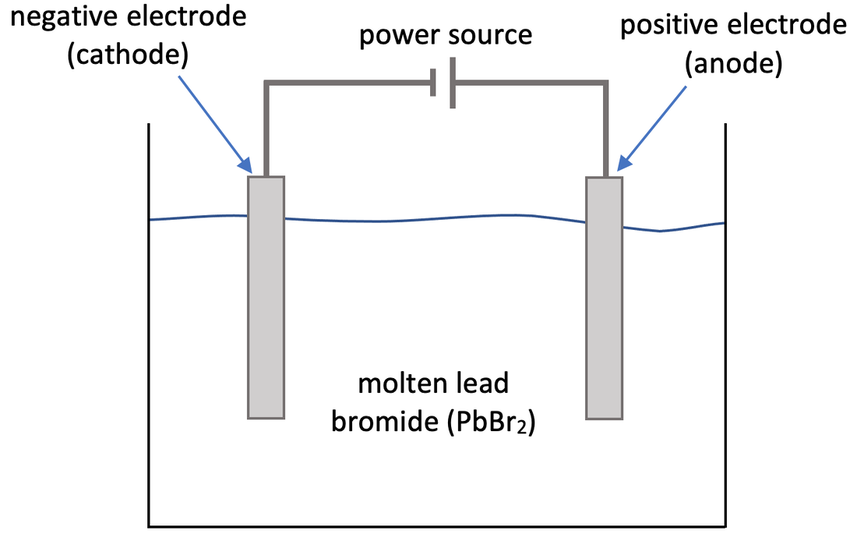
6) A student uses electrolysis to split up molten lead bromide (PbBr2). The step up of the student’s experiment is shown below.
a) Give the charges of the ions involved in lead bromide (PbBr2).
b)
i) Write the half equation for what happens at the cathode.
ii) Has this substance been oxidised or reduced? Explain your answer.
c)
i) Write the half equation for what happens at the anode.
ii) Has this substance been oxidised or reduced? Explain your answer.
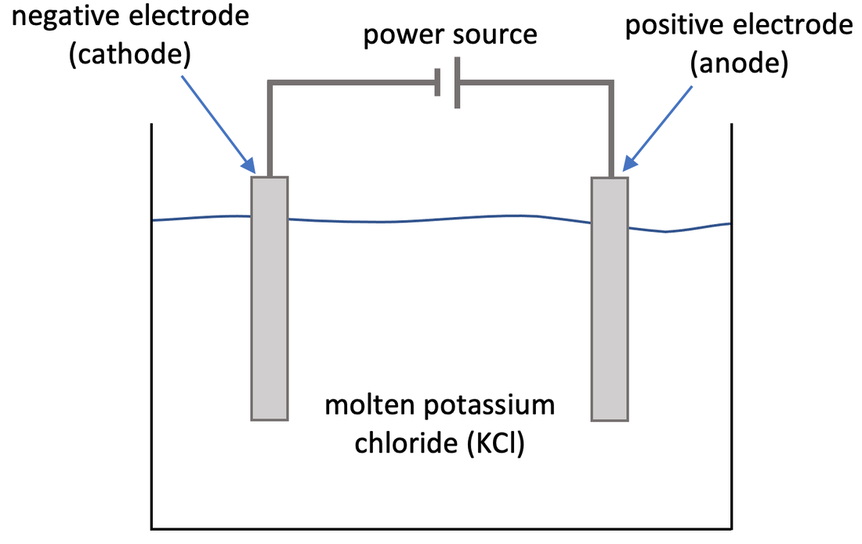
7) A student uses electrolysis to split up molten potassium chloride (KCl). The step up of the student’s experiment is shown below.
a) Give the charges of the ions involved in potassium chloride (KCl).
b) Write the half equation for what happens at the anode.
c) Write the half equation for what happens at the cathode.