1)
a) When the electrolyte for electrolysis is an aqueous solution, what additional ions do we get from the water in the aqueous solution?
b) Give the 4 ions that are present in an aqueous sodium chloride electrolyte. Sodium chloride is NaCl. You can either give the names or the charges.
c)
i) Is the cathode the positive or negative electrode?
ii) If the metal in the electrolyte is less reactive than hydrogen, what will happen at the cathode?
iii) If the metal in the electrolyte is more reactive than hydrogen, what will happen at the cathode?
d)
i) Is the anode the positive or negative electrode?
ii) If halide ions are present in the electrolyte, what will happen at the anode?
iii) If no halide ions are present in the electrolyte, what will happen at the anode?
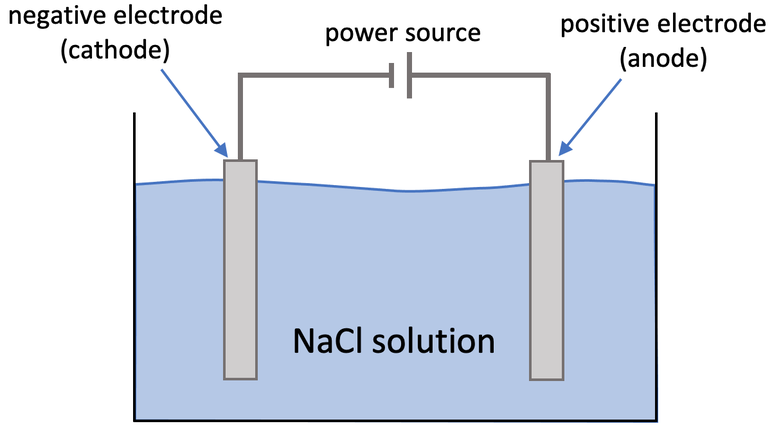
2) Aqueous sodium chloride can be electrolysed by using the apparatus below. Sodium chloride is NaCl.
a) A gas is produced at the cathode.
i) What gas is produced at the cathode?
ii) Write the half equation for the production of this gas.
iii) What is the test for this gas?
b) A gas is produced at the anode.
i) What gas is produced at the anode?
ii) Write the half equation for the production of this gas.
iii) What is the test for this gas?
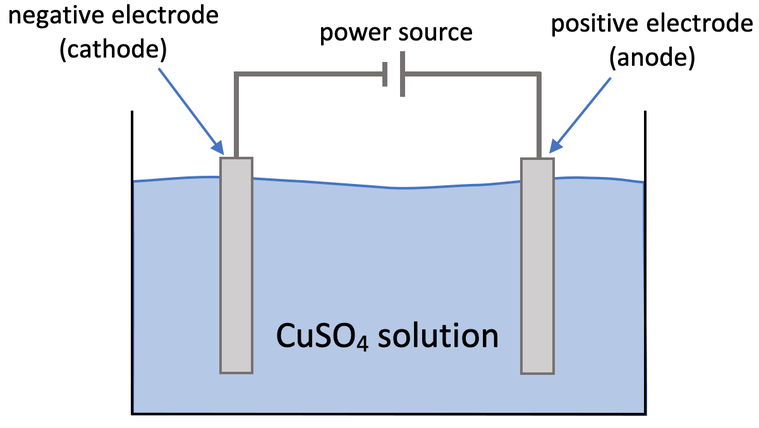
3) Aqueous copper sulfate (CuSO4) can be electrolysed by using the apparatus below.
a) Write the symbols for the 4 ions present in the electrolyte (2 of the ions come from copper sulfate and the other 2 come from the water).
b)
i) What will the student observe at the cathode? Explain why the student will observe this.
ii) Write the half equation for the cathode.
c) A gas and another product are produced at the anode.
i) What are the names of these two products produced at the anode?
ii) Write the half equation at the anode.
iii) What is the test for the gas produced at the anode?
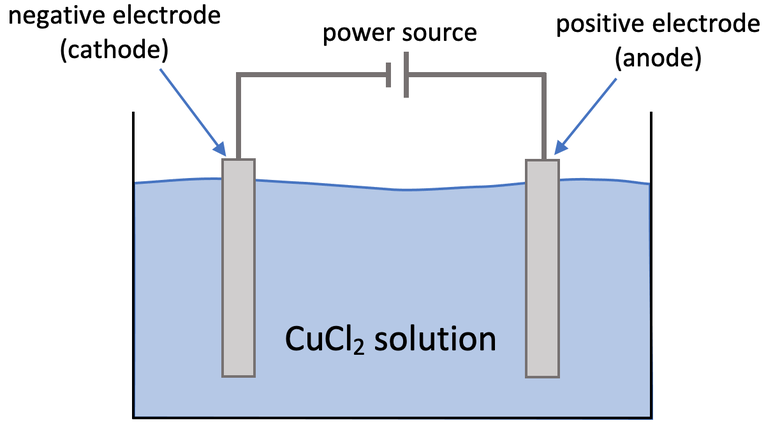
4) Aqueous copper chloride can be electrolysed by using the apparatus below. Copper chloride is CuCl2.
a)
i) What happens at the cathode? Explain why.
ii) Write the half equation for what happens at the cathode.
b)
i) What happens at the anode?
ii) Write the half equation for what happens at the anode.
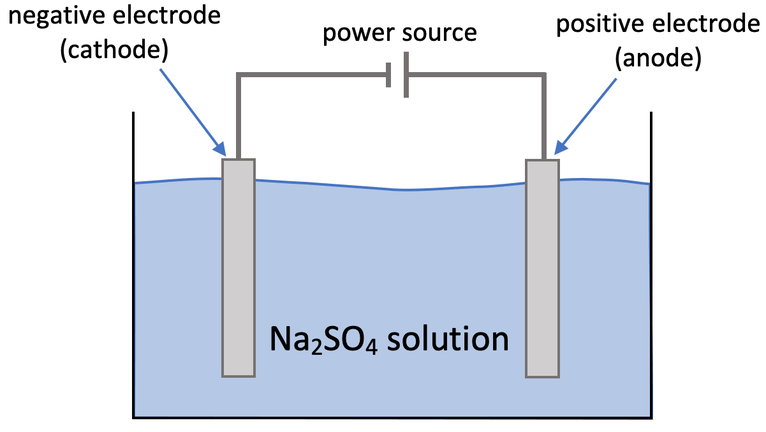
5) Aqueous sodium sulfate can be electrolysed by using the apparatus below. Sodium sulfate is Na2SO4.
a)
i) What happens at the cathode? Explain why.
ii) Write the half equation for what happens at the cathode.
b)
i) What happens at the anode? Explain why.
ii) Write the half equation for what happens at the anode.
6) Aqueous potassium bromide can be electrolysed by using the apparatus below. Potassium bromide is KBr.
a)
i) What happen at the anode? Explain why.
ii) Write the half equation for what happens at the anode.
b)
i) What happens at the cathode? Explain why.
ii) Write the half equation for what happens at the cathode.