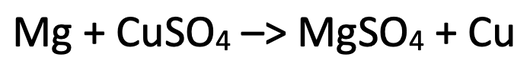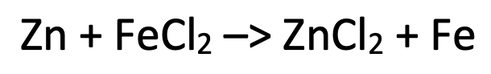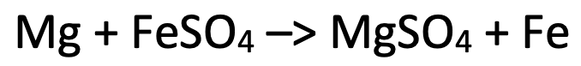Back to C4 Home
C4: Quiz 8
C4: Quiz 8
Click here for a PDF of the reaction equations in this quiz.
1) What does OIL RIG stand for?
2) The equation below shows the reaction between magnesium and copper sulfate.
1) What does OIL RIG stand for?
2) The equation below shows the reaction between magnesium and copper sulfate.
a) Out of magnesium and copper, which is the more reactive metal?
b)
i) Write this equation out in terms of the ions that are involved.
ii) Get rid of any ions that are the same on both sides of the equation.
iii) Write two half equations for the two metals.
iv) Which substance has been reduced? Explain your answer.
v) Which substance has been oxidised? Explain your answer.
3) The equation below shows the reaction between zinc and iron chloride.
b)
i) Write this equation out in terms of the ions that are involved.
ii) Get rid of any ions that are the same on both sides of the equation.
iii) Write two half equations for the two metals.
iv) Which substance has been reduced? Explain your answer.
v) Which substance has been oxidised? Explain your answer.
3) The equation below shows the reaction between zinc and iron chloride.
a) Write the ionic equation for this reaction (do not include any ions that are the same on both sides of the reaction).
b) Write two half equations for the metals.
c) Which substance has been reduced? Explain your answer.
d) Which substance has been oxidised? Explain your answer.
4) The equation below shows the reaction between magnesium and iron sulfate.
b) Write two half equations for the metals.
c) Which substance has been reduced? Explain your answer.
d) Which substance has been oxidised? Explain your answer.
4) The equation below shows the reaction between magnesium and iron sulfate.
a) Write the ionic equation for this reaction (do not include any ions that are the same on both sides of the reaction).
b) Write two half equations for the metals.
c) Which substance has been reduced? Explain your answer.
d) Which substance has been oxidised? Explain your answer.
5) Fill in the gaps in the sentences below with the words “oxidised”, “reduced”, “gains” and “loses”.
For metal displacement reactions, the metal that is doing the displacing is ______ as it ______ electron(s). And the metal that has been displaced is ______ as it ______ electron(s).
b) Write two half equations for the metals.
c) Which substance has been reduced? Explain your answer.
d) Which substance has been oxidised? Explain your answer.
5) Fill in the gaps in the sentences below with the words “oxidised”, “reduced”, “gains” and “loses”.
For metal displacement reactions, the metal that is doing the displacing is ______ as it ______ electron(s). And the metal that has been displaced is ______ as it ______ electron(s).