Back to C5 Home
C5 A) Endothermic & Exothermic Reactions
C5 A) Endothermic & Exothermic Reactions
Changes of energy occur when reactions take place. There are two different types of reactions; endothermic and exothermic reactions.
The easiest way to remember the difference between endothermic and exothermic reactions is to look at the start of the two words. For endothermic, the start is ‘en’ which is similar to in, so you can use this to remember that endothermic reactions take in energy. For exothermic, the start is ‘ex’ which is similar to exit, so you can use this to remember that energy exits for exothermic reactions.
We can draw reaction profiles for endothermic and exothermic reactions. Reaction profiles show the amount of energy in the reactants (which are the substances that are reacting) and the products (which are the substances produced by the reaction). Reaction profiles also show us how the energy changes during a reaction.
Reactions require a certain amount of energy to start. The amount of energy that is required to start a reaction is known as the activation energy and it varies for different reactions. The activation energy is the difference between the energy in the reactants and the highest energy point on the reaction profile. The activation energy for a reaction must be met otherwise the reaction will not take place. Both endothermic and exothermic reactions require energy to start the reaction, and this is why both of the reaction profiles for endothermic and exothermic reactions rise at the start during the reactions (the reaction profiles are shown below, so this comment will make more sense when you have seen both of the reaction profiles).
Reaction profiles also show us the overall energy change, which is the difference between the energy in the products and the energy in the reactants; it is “the energy in the products – the energy in the reactants”. We can have a positive or negative value for the overall energy change:
Let’s now have a look at the reaction profiles.
- Endothermic reactions take in energy from their surroundings. They usually take in energy in the form of heat, which means that the temperature of the surroundings will decrease after an endothermic reaction has taken place. Photosynthesis is an example of an endothermic reaction.
- Exothermic reactions give out energy to their surroundings. The energy given out is usually heat, which means that the temperature of the surroundings will increase after an exothermic reaction has taken place. Respiration or combustion are examples of exothermic reactions.
The easiest way to remember the difference between endothermic and exothermic reactions is to look at the start of the two words. For endothermic, the start is ‘en’ which is similar to in, so you can use this to remember that endothermic reactions take in energy. For exothermic, the start is ‘ex’ which is similar to exit, so you can use this to remember that energy exits for exothermic reactions.
We can draw reaction profiles for endothermic and exothermic reactions. Reaction profiles show the amount of energy in the reactants (which are the substances that are reacting) and the products (which are the substances produced by the reaction). Reaction profiles also show us how the energy changes during a reaction.
Reactions require a certain amount of energy to start. The amount of energy that is required to start a reaction is known as the activation energy and it varies for different reactions. The activation energy is the difference between the energy in the reactants and the highest energy point on the reaction profile. The activation energy for a reaction must be met otherwise the reaction will not take place. Both endothermic and exothermic reactions require energy to start the reaction, and this is why both of the reaction profiles for endothermic and exothermic reactions rise at the start during the reactions (the reaction profiles are shown below, so this comment will make more sense when you have seen both of the reaction profiles).
Reaction profiles also show us the overall energy change, which is the difference between the energy in the products and the energy in the reactants; it is “the energy in the products – the energy in the reactants”. We can have a positive or negative value for the overall energy change:
- A positive value for the overall energy change means that there is more energy in the products than the reactants. This means that the reaction has taken in energy, thus meaning that the reaction is endothermic.
- A negative value for the overall energy change means that there is less energy in the products than there was in the reactants. This means that the reaction gave out energy, thus meaning that the reaction is exothermic.
Let’s now have a look at the reaction profiles.
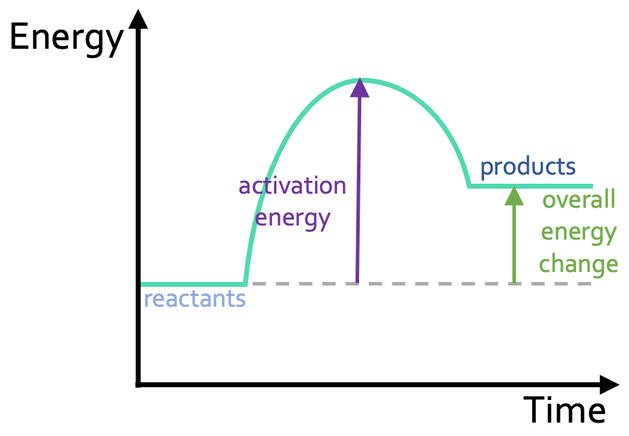
Endothermic Reaction Profile
Endothermic reactions take in energy from their surroundings. This means that the energy in the products is greater than the energy in the reactants; the line for the energy in the products (right line) will be higher than the line for the energy in the reactants (left line). The reaction profile for an endothermic reaction looks like what is shown below.
Endothermic reactions take in energy from their surroundings. This means that the energy in the products is greater than the energy in the reactants; the line for the energy in the products (right line) will be higher than the line for the energy in the reactants (left line). The reaction profile for an endothermic reaction looks like what is shown below.
On the above reaction profile, I have labelled the activation energy, which is the difference between the energy in the reactants (left line) and the highest energy point on the reaction profile. I have also labelled the overall energy change, which is the difference between the energy in the products and the energy in the reactants. For an endothermic reaction, the overall energy change is positive, and this is because the reaction has taken in energy (there is more energy in the products than the reactants).
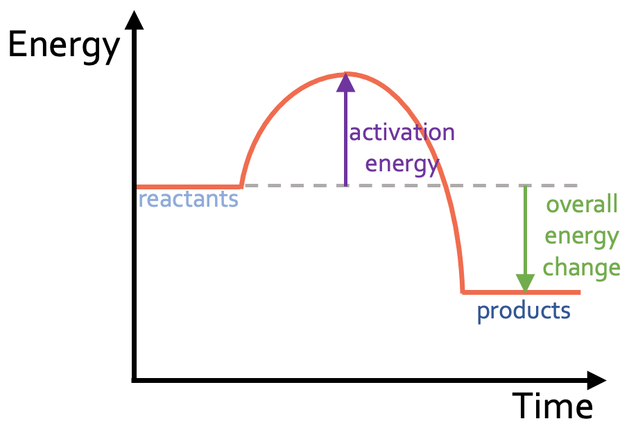
Exothermic Reaction Profile
Exothermic reactions give out energy to their surroundings. This means that the energy in the products is less than the energy in the reactants; the line for the energy in the products (right line) will be lower than the line for the energy in the reactants (left line). The reaction profile looks like what is shown below.
Exothermic reactions give out energy to their surroundings. This means that the energy in the products is less than the energy in the reactants; the line for the energy in the products (right line) will be lower than the line for the energy in the reactants (left line). The reaction profile looks like what is shown below.
On the above reaction profile, I have labelled the activation energy, which is the difference between the energy in the reactants (left line) and the highest energy point on the reaction profile. I have also labelled the overall energy change, which is the difference between the energy in the products and the energy in the reactants. For an exothermic reaction, the overall energy change is negative, and this is because the reaction has given out energy (there is less energy in the products than the reactants).