Back to C6 Home
C6: Quiz 4 – Answers
C6: Quiz 4 – Answers
1)
a) 1.83 cm3/s (±0.1)
b) 0.71 cm3/s (±0.1)
c) 14 to 16 seconds
d) 0.87 to 1 cm3/s
2)
a)
b) Anytime between 26 and 30 seconds
c) 0.43-0.5 g/s
d) 0.41 g/s (±0.05)
e) 0.21 g/s (±0.05)
3)
a)
i) The tangent at 2 seconds (the graph also shows the tangent at 15 seconds for part b)
ii) 1.8 g/s (±0.2)
b) 0.35 g/s (±0.1)
4)
a)
i) The tangent at 2.5 seconds (the graph also shows the tangent at 20 seconds for part b)
ii) 1.6 cm3/s (±0.2)
b) 0.18 cm3/s (±0.1)
Questions
Click here for a printable PDF of the graphs in this quiz.
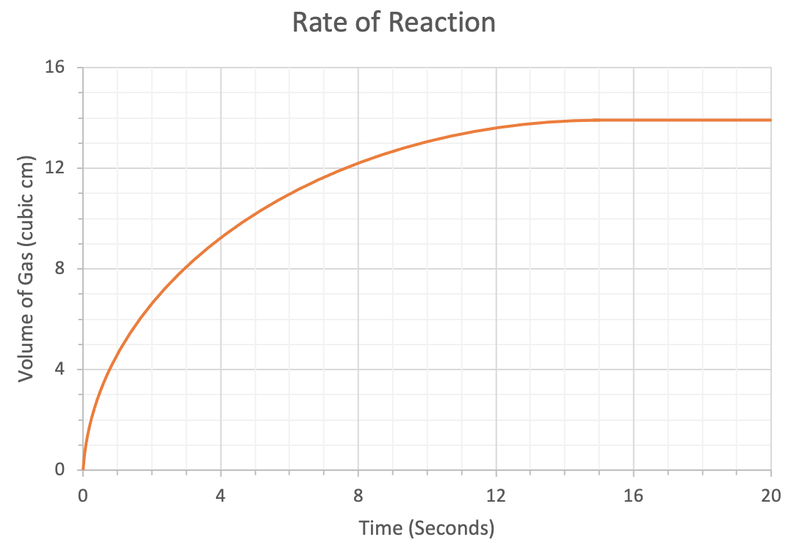
1) The rate of reaction graph below shows the volume of gas produced over time.
Click here for a printable PDF of the graphs in this quiz.
1) The rate of reaction graph below shows the volume of gas produced over time.
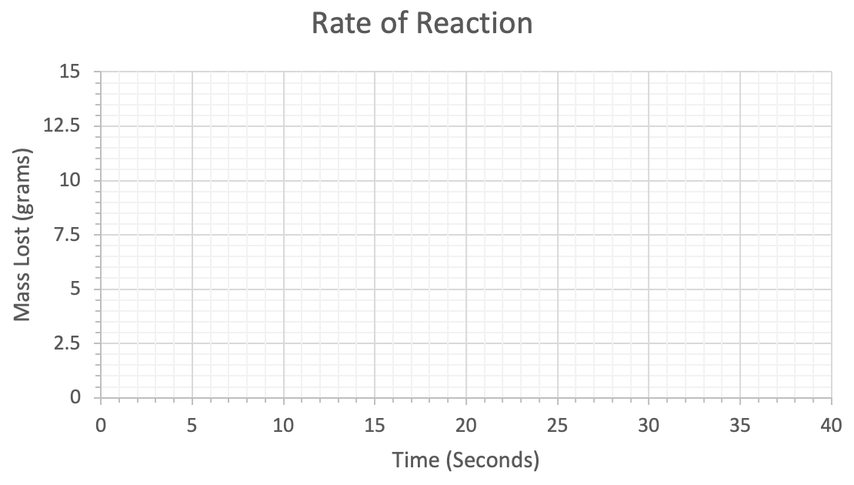
a) Work out the mean rate of reaction between 0 and 6 seconds. Give your answer to 2 decimal places and include the units in your answer.
b) Work out the mean rate of reaction between 3 and 10 seconds. Give your answer to 2 decimal places and include the units in your answer.
c) When does the reaction end?
d) Calculate the mean rate of reaction for this whole reaction. Give your answer to 2 decimal places and include the units in your answer.
2) Parts a and b for this question appeared in the previous quiz. Therefore, feel free to use the graph that you drew in the previous quiz rather than drawing the graph again.
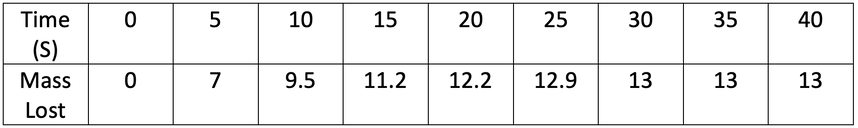
A student investigated the rate of reaction by measuring mass lost against time. His results are shown in the table below.
b) Work out the mean rate of reaction between 3 and 10 seconds. Give your answer to 2 decimal places and include the units in your answer.
c) When does the reaction end?
d) Calculate the mean rate of reaction for this whole reaction. Give your answer to 2 decimal places and include the units in your answer.
2) Parts a and b for this question appeared in the previous quiz. Therefore, feel free to use the graph that you drew in the previous quiz rather than drawing the graph again.
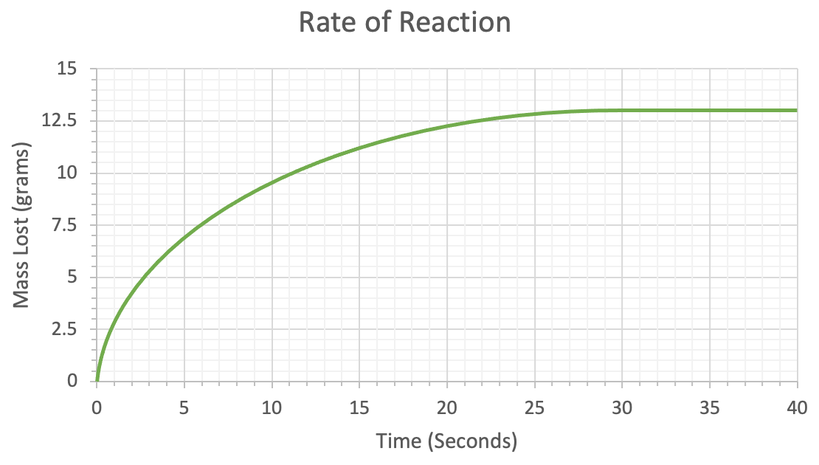
A student investigated the rate of reaction by measuring mass lost against time. His results are shown in the table below.
a) Plot his results on the graph below.
b) At what time does the reaction stop?
c) Calculate the mean rate of reaction for the whole reaction. Give your answer to 2 decimal places and include the units in your answer.
d) Calculate the mean rate of reaction between 5 and 16 seconds. Give your answer to 2 decimal places and include the units in your answer.
e) Calculate the mean rate of reaction between 14 and 21 seconds. Give your answer to 2 decimal places and include the units in your answer.
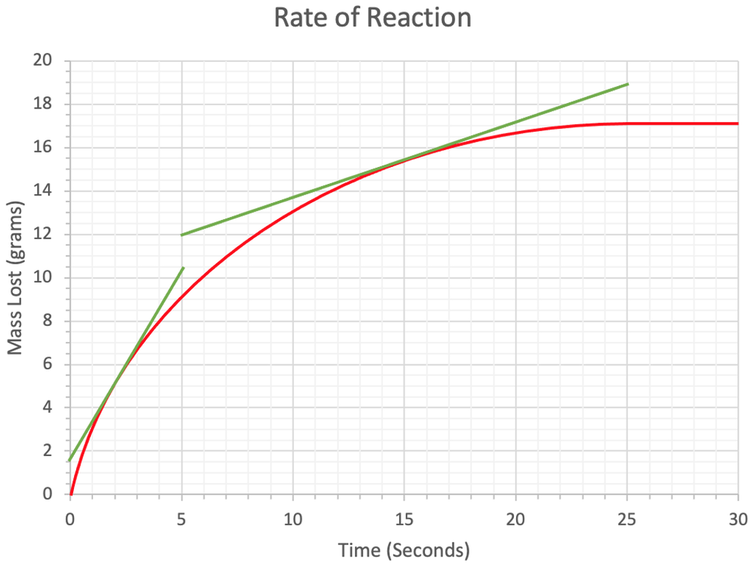
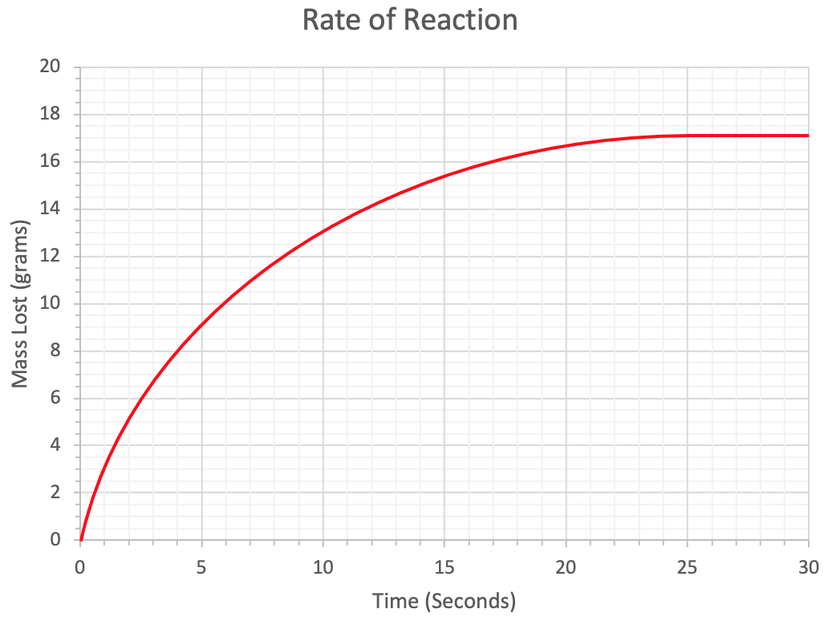
3) We have the rate of reaction graph below.
c) Calculate the mean rate of reaction for the whole reaction. Give your answer to 2 decimal places and include the units in your answer.
d) Calculate the mean rate of reaction between 5 and 16 seconds. Give your answer to 2 decimal places and include the units in your answer.
e) Calculate the mean rate of reaction between 14 and 21 seconds. Give your answer to 2 decimal places and include the units in your answer.
3) We have the rate of reaction graph below.
a) The subparts for part a will help you find the rate of reaction at a particular time.
i) Draw a tangent to the curve at 2 seconds.
ii) Use your tangent to work out the rate of reaction at 2 seconds. Give your answer to 2 significant figures and include the units in your answer.
b) Work out the rate of reaction at 15 seconds. Give your answer to 2 significant figures and include the units in your answer.
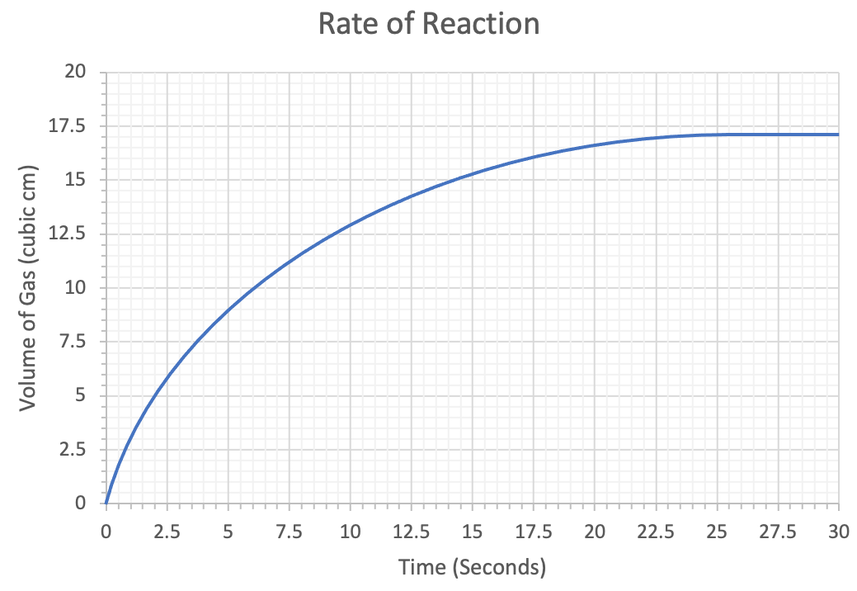
4) We have the rate of reaction graph below.
i) Draw a tangent to the curve at 2 seconds.
ii) Use your tangent to work out the rate of reaction at 2 seconds. Give your answer to 2 significant figures and include the units in your answer.
b) Work out the rate of reaction at 15 seconds. Give your answer to 2 significant figures and include the units in your answer.
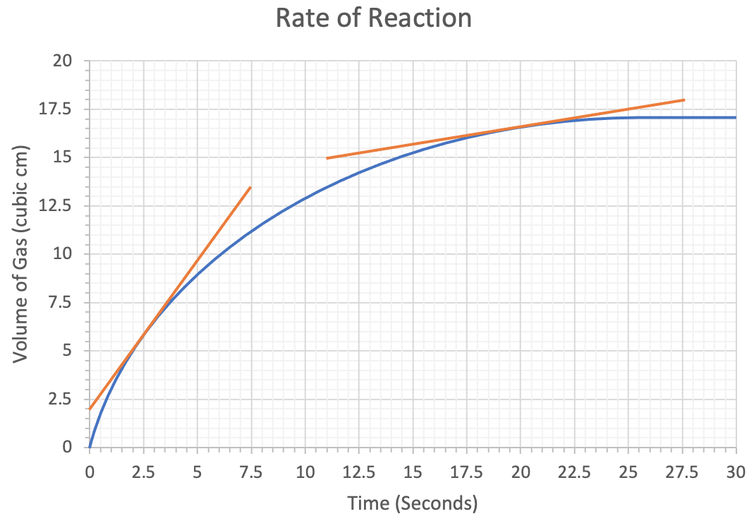
4) We have the rate of reaction graph below.
a) The subparts for part a will help us find the rate of reaction at a particular point.
i) Draw a tangent to the curve at 2.5 seconds.
ii) Use your tangent to work out the rate of reaction at 2.5 seconds. Give your answer to 2 significant figures and include the units in your answer.
b) Work out the rate of reaction at 20 seconds. Give your answer to 2 significant figures and include the units in your answer.
i) Draw a tangent to the curve at 2.5 seconds.
ii) Use your tangent to work out the rate of reaction at 2.5 seconds. Give your answer to 2 significant figures and include the units in your answer.
b) Work out the rate of reaction at 20 seconds. Give your answer to 2 significant figures and include the units in your answer.