1) The nuclear model
2)
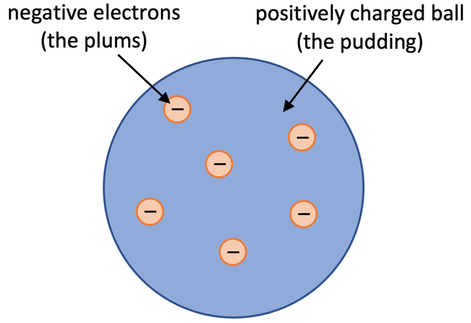
a) The plum pudding model stated that atoms are balls of positive charge with negative electrons embedded in the ball of positive charge. In the plum pudding model, the plums are the electrons and the pudding is the positively charged ball.
b)
2)
a) The plum pudding model stated that atoms are balls of positive charge with negative electrons embedded in the ball of positive charge. In the plum pudding model, the plums are the electrons and the pudding is the positively charged ball.
b)
3)
a) An alpha particle is a helium nuclei; it contains 2 protons and 2 neutrons
b) The plum pudding model predicted that the majority of the alpha particles would go straight through the sheet of gold foil or only be slightly deflected.
c) However, Rutherford and Marsden observed that:
- most of the alpha particles passed straight through as expected
- some of the alpha particles passed through the foil and were deflected more than expected
- a small number of the alpha particles were deflected back from where they came from, which was not expected
e) The positively charged nucleus of the gold atoms explains why some of the alpha particles were deflected more than expected. This is because the alpha particles are positive and so is the small nuclei of the gold atoms. Whenever an alpha particle passed near to the nucleus of a gold atom, it would be deflected because the same charges repel one another; the positively charged gold nuclei and positively charged alpha particle would repel each other, which will result in the alpha particle having its pathway changed. Whenever the alpha particle was fired directly at the nuclei of a gold atom, the alpha particle would be deflected backwards towards the source that was emitting the alpha particles for the same reason; the positively charged gold nuclei would repel the positively charged alpha particle.
4)
a) Bohr’s nuclear model suggested that the electrons orbit the nucleus in shells. Each of the shells are a fixed distance from the positively charged nuclei of the atom
b) Scientists thought that in Rutherford’s model of the atom, the negative electrons that are in the cloud around the nucleus would be attracted to the positively charged nucleus because opposite charges attract. This would cause the atom to collapse. Therefore, Bohr and other scientists concluded that the model of the atom needed to be modified slightly
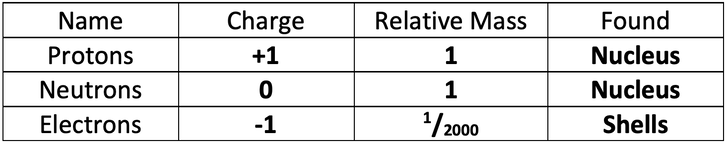
5) Name: neutron
Charge: neutral
6)
7)
a) If an electron absorbs EM radiation, they move to a higher energy level that is further away from the nucleus (they move to a shell that is further out)
b) If an electrons releases EM radiation, they move to a lower energy level that is closer to the nucleus (they move to a shell that is closer to the nucleus).
Questions
1) There are many different models of atoms. What is the name of the current model of the atom that nearly all scientists agree with?
2)
a) Describe the plum pudding model.
b) Draw a diagram for the plum pudding model.
3) Ernest Rutherford and Marsden completed an experiment where they fired alpha particles at a sheet of gold foil.
a) What is an alpha particle?
b) What were the predictions of the outcome of the experiment according to the plum pudding model?
c) What did Rutherford and Marsden observe when they completed their experiment?
d) Their observations went against the plum pudding model. How did Rutherford change the model of the atom to account for these observations?
e) How does Rutherford’s modifications to the model of the atom explain what they observed?
4)
a) How did Bohr modify Rutherford’s model?
b) Why did he/ scientists believe that Rutherford’s model needed to be changed?
5) James Chadwick discovered that there was another subatomic particle that atoms were made from. What was the name and charge of this subatomic particle that he discovered?
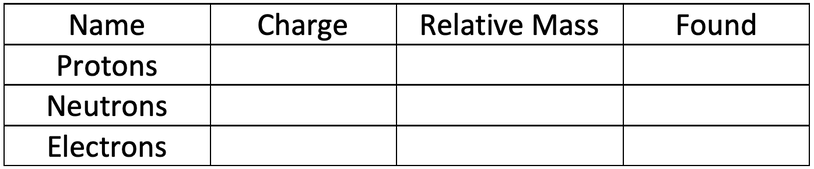
6) The current model that we use is the nuclear model. Fill in the table below for the charges, mass and found (location within the atom) for the three different subatomic particles.
1) There are many different models of atoms. What is the name of the current model of the atom that nearly all scientists agree with?
2)
a) Describe the plum pudding model.
b) Draw a diagram for the plum pudding model.
3) Ernest Rutherford and Marsden completed an experiment where they fired alpha particles at a sheet of gold foil.
a) What is an alpha particle?
b) What were the predictions of the outcome of the experiment according to the plum pudding model?
c) What did Rutherford and Marsden observe when they completed their experiment?
d) Their observations went against the plum pudding model. How did Rutherford change the model of the atom to account for these observations?
e) How does Rutherford’s modifications to the model of the atom explain what they observed?
4)
a) How did Bohr modify Rutherford’s model?
b) Why did he/ scientists believe that Rutherford’s model needed to be changed?
5) James Chadwick discovered that there was another subatomic particle that atoms were made from. What was the name and charge of this subatomic particle that he discovered?
6) The current model that we use is the nuclear model. Fill in the table below for the charges, mass and found (location within the atom) for the three different subatomic particles.
7)
a) If an electron absorbs electromagnetic radiation, what happens to the electron?
b) If an electron emits electromagnetic radiation, what happens to the electron?