C1: Quiz 13 – Answers
1) Halogens
2)
a) 7 electrons in their outermost shell
b) 1 electron off having a full outer shell of electrons
3)
a) The travel around in pairs, for example F2, Cl2 and Br2
b) Covalent bonds
4)
a) They all have low melting and boiling points as they have weak intermolecular forces of attraction, which require little energy to break
b) The melting and boiling points for halogens increase as we go down the group 7 column. This is because the relative atomic/ molecular masses increase as we go down the group 7 column, which increases the intermolecular forces of attraction, which requires more energy to break, hence the higher melting and boiling points for halogens that are further down the group 7 column.
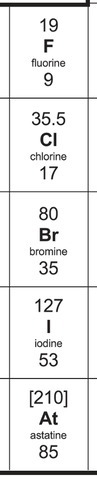
5) The reactivity of halogens decreases as we go down the group 7 column; fluorine is the most reactive and astatine is the least reactive. This is because higher up group 7 elements are trying to gain an electron that is on a shell that is closer to the positively charged nucleus. This means that there is a stronger force of attraction for higher up group 7 elements between the positively charged nucleus and the electron that the group 7 element is trying to gain. This stronger force of attraction means that higher up group 7 elements can attract an electron more easily, thus meaning that the group 7 elements at the top are the most reactive
6) The charge is 1-, F- or F1-. This charge comes about because fluorine gains/ receives 1 electron from the metal resulting in fluorine having a negative charge of 1 (1-)
7)
a) Fluorine is a poisonous yellow gas
b) Chlorine is a poisonous dense green gas
c) Bromine is a poisonous dense red-brown-orange liquid
d) Iodine is a grey solid or purple vapour
1) What is another name for the group 7 elements?
2)
a) How many electrons do the group 7 elements have in their outermost shell?
b) How many electrons are the group 7 elements off having a full outer shell?
3)
a) Halogens travel around as diatomic molecules; what does that mean?
b) What are the bonds involved in the diatomic molecules?
4)
a) With respect to all of the other elements in the periodic table, do all group 7 elements have relatively high or relatively low melting and boiling points? Explain your answer.
b) Do the melting and boiling points of the halogens increase or decrease as we go down the group 7 column? Explain your answer.
5) The halogens in the periodic table are shown below.
6) Halogens can react with metals to produce halide ions. What would be the charge on a fluoride ion and how would this charge come about?
7) What colours and states are the following halogens at room temperature?
a) Fluorine
b) Chlorine
c) Bromine
d) Iodine