Back to C2 Home
C2: Quiz 10
C2: Quiz 10
1) If H2O was to change state from solid (ice) to liquid (water), would that be a physical or chemical change? Explain your answer.
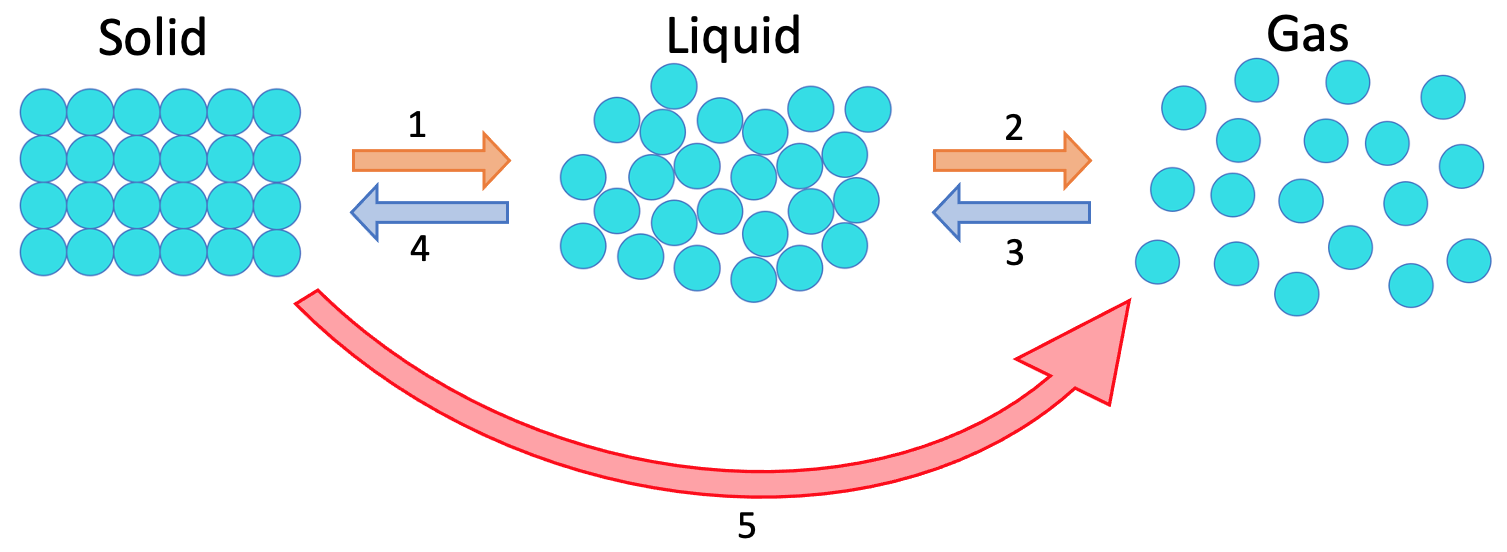
2) The diagram below shows the three different states of matter.
What are the names for the 5 different changes of state on the diagram above?
3) Explain how a substance changes state from a solid to a liquid.
4) Explain how a substance changes state from a gas to a liquid.
5) Ethanol has a melting point of -114°C and a boiling point of 78°C. Predict the states for ethanol at the following temperatures:
a) 10°C
b) 178°C
c) -120°C
6) Sodium chloride has a melting point of 801°C and a boiling point of 1,465°C. Predict the states for sodium chloride at the following temperatures:
a) 175°C
b) 2500°C
c) -120°C
d) 1,200°C
7) Fluorine has a melting point of -220°C and a boiling point of -188°C. Predict the states for fluorine at the following temperatures:
a) 160°C
b) -200°C
c) -67°C
d) -251°C
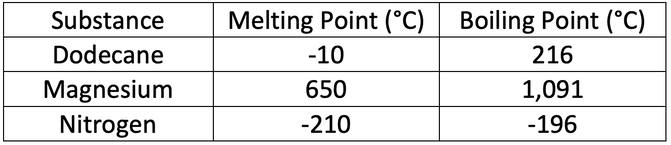
8) The table below shows the melting and boiling points of three different substances.
3) Explain how a substance changes state from a solid to a liquid.
4) Explain how a substance changes state from a gas to a liquid.
5) Ethanol has a melting point of -114°C and a boiling point of 78°C. Predict the states for ethanol at the following temperatures:
a) 10°C
b) 178°C
c) -120°C
6) Sodium chloride has a melting point of 801°C and a boiling point of 1,465°C. Predict the states for sodium chloride at the following temperatures:
a) 175°C
b) 2500°C
c) -120°C
d) 1,200°C
7) Fluorine has a melting point of -220°C and a boiling point of -188°C. Predict the states for fluorine at the following temperatures:
a) 160°C
b) -200°C
c) -67°C
d) -251°C
8) The table below shows the melting and boiling points of three different substances.
You may get more than one substance for each of the following questions.
a) Which of the substances will be a solid at 0°C
b) Which of the substances will be a gas at -150°C
c) Which of the substances will be a liquid at 85°C
d) Which of the substances will be a gas at 1,200°C
a) Which of the substances will be a solid at 0°C
b) Which of the substances will be a gas at -150°C
c) Which of the substances will be a liquid at 85°C
d) Which of the substances will be a gas at 1,200°C