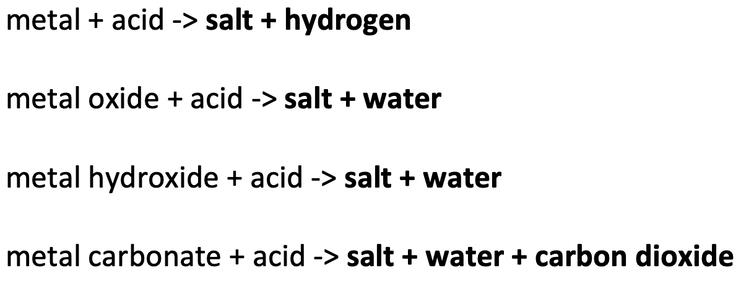C4: Quiz 3 – Answers
2)
3)
a) Collect some of the potential hydrogen gas in a test tube and place a lit splint into the test tube. If you hear a squeaky pop, hydrogen is present
b) Bubble the potential carbon dioxide gas through lime water (lime water is calcium hydroxide dissolved in water). If carbon dioxide is present, the limewater will turn cloudy
4)
a) magnesium + hydrochloric acid –> magnesium chloride + hydrogen
b) Mg + 2 HCl –> MgCl2 + H2
5)
a) zinc + sulfuric acid –> zinc sulfate + hydrogen
b) Zn + H2SO4 –> ZnSO4 + H2
6)
a) copper oxide + hydrochloric acid –> copper chloride + water
b) CuO + 2 HCl –> CuCl2 + H2O
7)
a) potassium hydroxide + nitric acid –> potassium nitrate + water
b) KOH + HNO3 –> KNO3 + H2O
8)
a) CO3
b) 2- or -2 or CO32-
9)
a) calcium carbonate + hydrochloric acid –> calcium chloride + water + carbon dioxide
b) CaCO3 + 2 HCl –> CaCl2 + H2O + CO2
10)
a) sodium carbonate + sulfuric acid –> sodium sulfate + water + carbon dioxide
b) Na2CO3 + H2SO4 –> Na2SO4 + H2O + CO2
11)
a) aluminium + hydrochloric acid –> aluminium chloride + hydrogen
b)
i) 3+ or +3 or Al3+
ii) - or 1- or -1 or Cl-
iii) AlCl3
c) 2 Al + 6 HCl –> 2 AlCl3 + 3 H2
Click here for a printable PDF of question 1 & question 2 for this quiz.
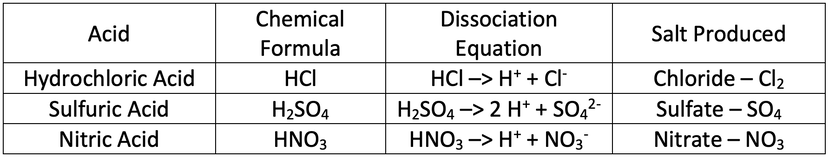
1) Complete the table below.
2) Write the products for the following reactions.
3)
a) What is the test for hydrogen?
b) What is the test for carbon dioxide?
4)
a) Write the word equation for the reaction of magnesium with hydrochloric acid.
b) Write the balanced chemical equation for this reaction.
5)
a) Write the word equation for the reaction of zinc with sulfuric acid.
b) Write the balanced chemical equation for this reaction.
6)
a) Write the word equation for the reaction of copper oxide with hydrochloric acid.
b) Write the balanced chemical equation for this reaction.
7)
a) Write the word equation for the reaction of potassium hydroxide with nitric acid.
b) Write the balanced chemical equation for this reaction.
8)
a) What is the chemical symbol for a carbonate?
b) What is the charge on a carbonate ion?
9)
a) Write the word equation for the reaction of calcium carbonate with hydrochloric acid.
b) Write the balanced chemical equation for this reaction.
10)
a) Write the word equation for the reaction of sodium carbonate with sulfuric acid.
b) Write the balanced chemical equation for this reaction.
11) This last question is very tricky.
a) Write the word equation for the reaction of aluminium with hydrochloric acid.
b)
i) When aluminium ionically bonds, what will the charge be on an aluminium ion?
ii) When chlorine ionically bonds, what will the charge be on a chloride ion?
iii) Hence or otherwise, what is the chemical formula for aluminium chloride?
c) Write the balanced chemical equation for this reaction.