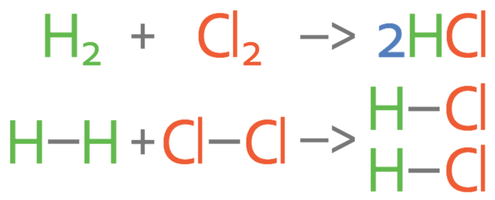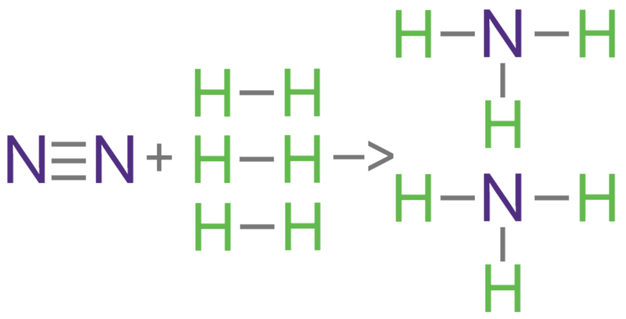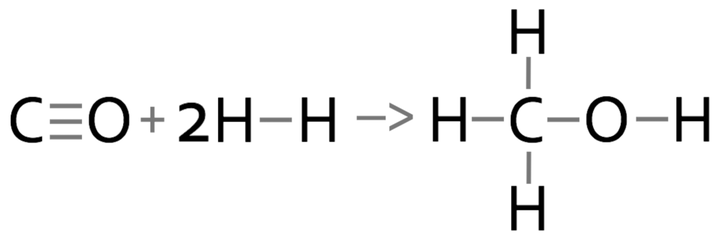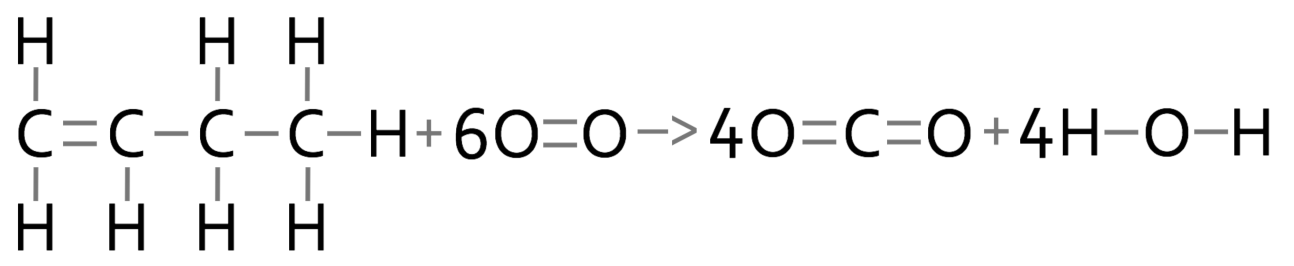Back to C5 Home
C5: Quiz 2
C5: Quiz 2
Click here for a printable PDF of the equations and tables in this quiz. The first question was one of the questions that we looked at in the written content. Therefore, feel free to skip this one if you were okay with it.
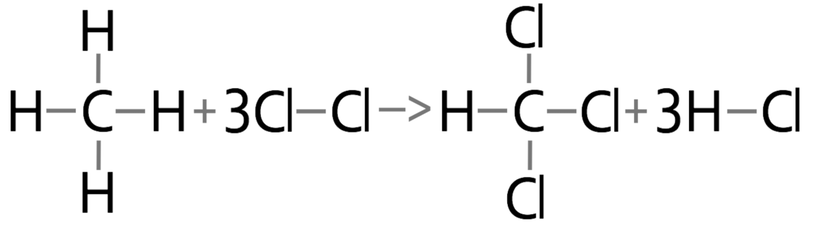
1) Hydrogen and chlorine react to produce hydrogen chlorine gas.
1) Hydrogen and chlorine react to produce hydrogen chlorine gas.
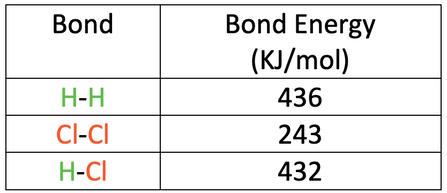
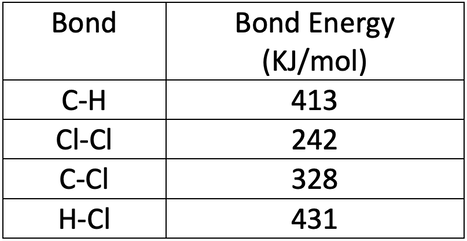
The bond energies are shown in the table below.
a) Calculate the overall energy change for this reaction.
b) Is this reaction exothermic or endothermic? Give a reason for your answer.
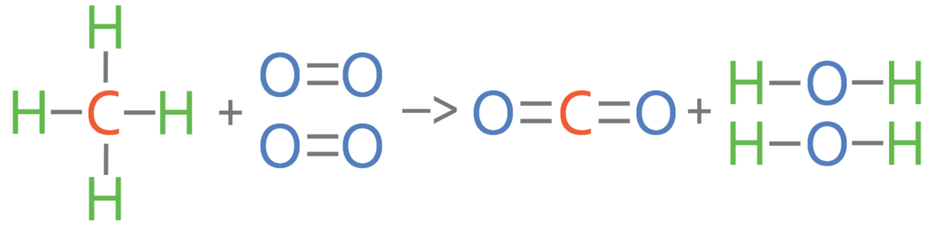
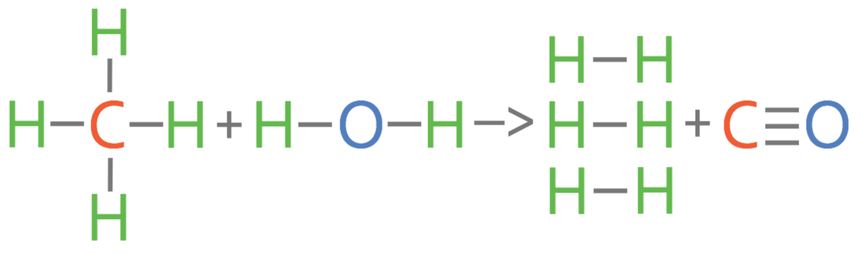
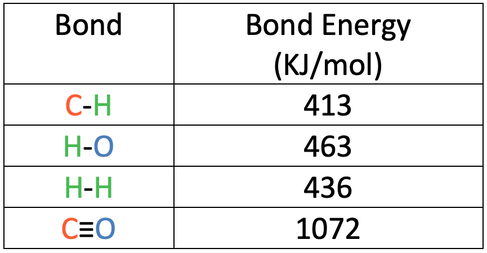
2) Methane reacts with oxygen to produce carbon dioxide and water.
b) Is this reaction exothermic or endothermic? Give a reason for your answer.
2) Methane reacts with oxygen to produce carbon dioxide and water.
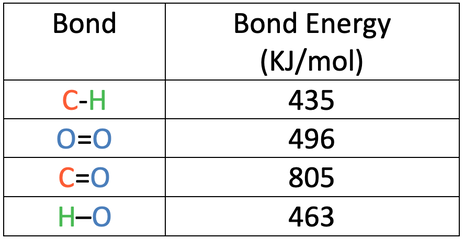
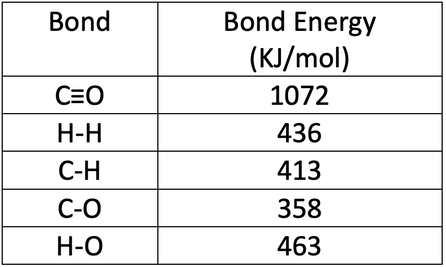
The bond energies are shown in the table below.
a) Calculate the overall energy change for this reaction.
b) Is this reaction exothermic or endothermic? Give a reason for your answer.
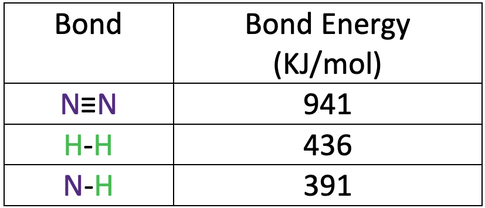
3) Nitrogen reacts with hydrogen to produce ammonia.
b) Is this reaction exothermic or endothermic? Give a reason for your answer.
3) Nitrogen reacts with hydrogen to produce ammonia.
The bond energies are shown in the table below.
a) Calculate the overall energy change for this reaction.
b) Is this reaction exothermic or endothermic?
4) The reaction of methane with water produces hydrogen and carbon monoxide.
b) Is this reaction exothermic or endothermic?
4) The reaction of methane with water produces hydrogen and carbon monoxide.
The bond energies are shown in the table below.
a) Calculate the overall energy change for this reaction.
b) Is this reaction exothermic or endothermic? Give a reason for your answer.
For the next few questions, I am just going to show you what one of the molecules looks like. For example, in question 5, I am just going to show you what one hydrogen molecule looks like and not what two of them look like. Therefore, just be careful with the following questions.
5) We have the reaction below.
b) Is this reaction exothermic or endothermic? Give a reason for your answer.
For the next few questions, I am just going to show you what one of the molecules looks like. For example, in question 5, I am just going to show you what one hydrogen molecule looks like and not what two of them look like. Therefore, just be careful with the following questions.
5) We have the reaction below.
The bond energies are shown in the table below.
Calculate the overall energy change for this reaction and say whether the reaction is endothermic or exothermic.
6) We have the reaction below.
6) We have the reaction below.
The bond energies are shown in the table below.
Calculate the overall energy change for this reaction and say whether the reaction is endothermic or exothermic.
7) The reaction below is the combustion of butene in air.
7) The reaction below is the combustion of butene in air.
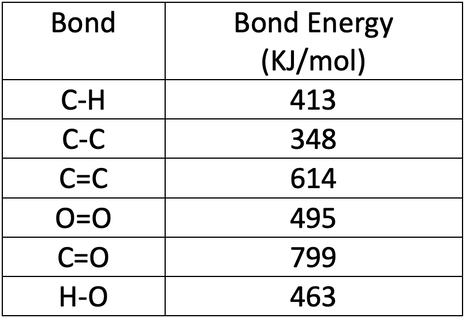
The bond energies are shown in the table below.
Calculate the overall energy change for this reaction and say whether the reaction is endothermic or exothermic.
Be very careful with butene because there are two types of carbon-carbon bonds
Be very careful with butene because there are two types of carbon-carbon bonds