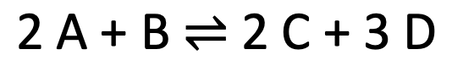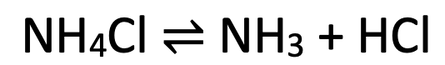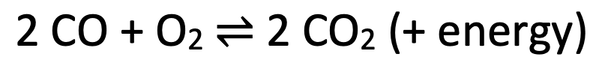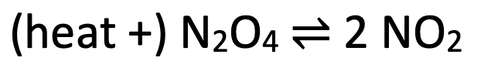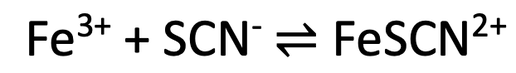C6: Quiz 7
a) What is a dynamic equilibrium?
b) Fill in the gap in the following sentence; a dynamic equilibrium can only occur in a __________ __________?
c) Are reactions still taking place at a dynamic equilibrium; yes or no?
2) What are the 3 factors that affect the position of a dynamic equilibrium?
3) Le Chatelier says that if we change any of the 3 factors that are the answer to question 2, the reversible reaction will respond by counteracting the change.
a) Fill in the gaps below with either “increasing”, “decreasing”, “endothermic” or “exothermic”.
If we increase the temperature, the reversible reaction will respond by _______ the temperature. This means that there will be more _______ reactions, and fewer _______ reactions.
b) Fill in the gaps below with either “increasing”, “decreasing”, “fewer” or “more”.
If we increase the pressure, the reversible reaction will respond by _______ the pressure. This moves the position of the equilibrium towards the side of the reversible reaction with _______ molecules/ moles of gas.
c) Fill in the gap below with either “reactants” or “products”.
If we increase the concentration of one of the reactants, the reversible reaction will respond by making more of the _______.
4) We have the reversible reaction below.
a) Is the backwards reaction endothermic or exothermic?
b) We now increase the temperature of the system. In which direction will the position of the dynamic equilibrium move? Explain your answer.
c) We now increase the pressure of the system. In which direction will the position of the dynamic equilibrium move? Explain your answer.
d) We now decrease the concentration of C & D in the system. In which direction will the position of the dynamic equilibrium move? Explain your answer.
5) When we heat ammonium chloride, it breaksdown into ammonia and hydrogen chloride. This reaction is a reversible reaction and it is taking place in a closed container. The reversible reaction is:
b) We now decrease the temperature of the system. In which direction will the position of the dynamic equilibrium move? Explain your answer.
c) We now decrease the pressure of the system. In which direction will the position of the dynamic equilibrium move? Explain your answer.
6) The reaction of carbon monoxide and oxygen to produce carbon dioxide is a reversible reaction. The reversible reaction is shown below.
I want to move the dynamic equilibrium as far towards the right as possible; I want as much carbon dioxide as possible.
a) Should I use a low or high temperature? Explain your answer.
b) Should I use a low or high pressure? Explain your answer.
7) Dinitrogen tetroxide (N2O4) can decompose to produce nitrogen dioxide (NO2). The reversible reaction for this is shown below.
Dinitrogen tetroxide (N2O4) is a colourless gas and nitrogen dioxide (NO2) is a brown gas.
a) Assume that the dynamic equilibrium is in the middle.
i) If the dynamic equilibrium moved towards the right (more NO2), how would you expect the colour of the equilibrium mixture to change; would it become lighter brown or darker brown?
ii) If the dynamic equilibrium moved towards the left (more N2O4), how would you expect the colour of the equilibrium mixture to change; would it become lighter brown or darker brown?
b) We are now going to decrease the temperature of the vessel that the reaction is taking place in. Predict the colour change of the equilibrium mixture; would it become lighter brown or darker brown? Explain your answer.
c) We are now going to increase the pressure of the vessel that the reaction is taking place in. Predict the colour change of the equilibrium mixture; would it become lighter brown or darker brown? Explain your answer.
8) Iron (III) ions (Fe3+) can react with thiocyanate ions (SCN-) to produce iron thiocyanate ions (FeSCN2+). All 3 of these substances are in a solution (aqueous). This reaction is reversible and it is shown below.
Iron (III) ions are yellow, thiocyanate ions are colourless and iron thiocyanate ions are reddish-brown. The reaction is going to take place in a closed system.
a) If the position of the dynamic equilibrium was on the left, what would the colour of the equilibrium mix be?
b) If the position of the dynamic equilibrium was on the right, what would the colour of the equilibrium mix be?
c) A student increases the concentration of iron (III) ions (Fe3+).
i) Describe the colour change immediately after the iron (III) ions (Fe3+) are added.
ii) Describe the colour change when the new dynamic equilibrium has been reached. Explain your answer.