Back to C7 Home
C7: Quiz 3
C7: Quiz 3
Click here for a printable PDF of the graphs in this quiz.
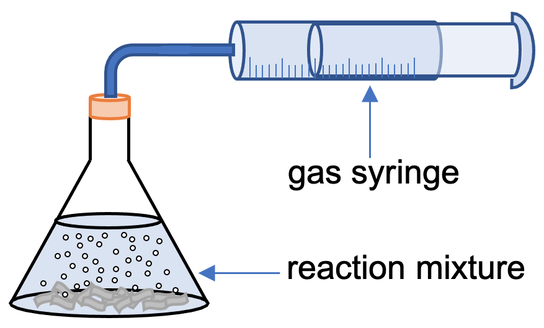
1) A student investigates how temperature affects the rate of reaction between a given mass of magnesium strips and some acid. The student sets up the apparatus like what is shown below.
1) A student investigates how temperature affects the rate of reaction between a given mass of magnesium strips and some acid. The student sets up the apparatus like what is shown below.
The student measured the volume of gas produced over time. He completes the experiment/ reaction at 30°C, 60°C and 90°C.
a) What is the independent variable in this experiment?
b) What is the dependent variable in this experiment?
c) Give two variables that need to be controlled during this experiment.
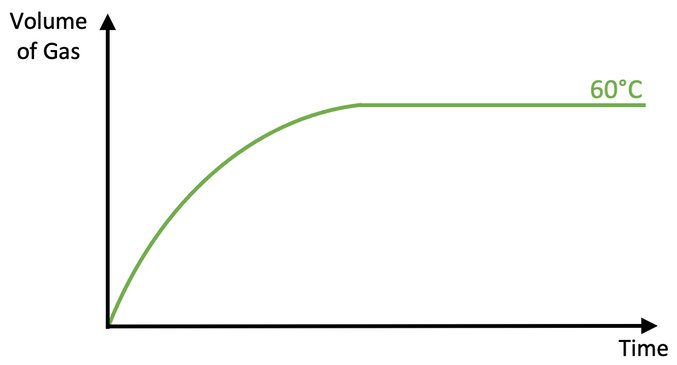
The student then draws a graph for the volume of gas produced against time for the reaction at 60°C.
a) What is the independent variable in this experiment?
b) What is the dependent variable in this experiment?
c) Give two variables that need to be controlled during this experiment.
The student then draws a graph for the volume of gas produced against time for the reaction at 60°C.
d)
i) Is the rate of reaction at 90°C faster or slower than the rate of reaction at 60°C? Use collision theory to explain your answer.
ii) Add a curve to the above graph for the reaction at 90°C.
e) Add a curve to the above graph for the reaction at 30°C.
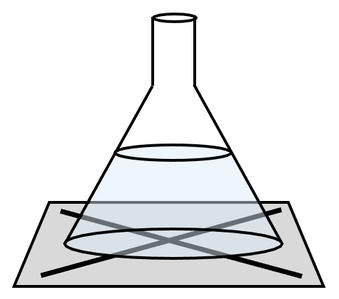
2) A student undertakes a precipitation reaction to see what effect concentration has on the rate of reaction. She sets up the apparatus like what is shown below.
i) Is the rate of reaction at 90°C faster or slower than the rate of reaction at 60°C? Use collision theory to explain your answer.
ii) Add a curve to the above graph for the reaction at 90°C.
e) Add a curve to the above graph for the reaction at 30°C.
2) A student undertakes a precipitation reaction to see what effect concentration has on the rate of reaction. She sets up the apparatus like what is shown below.
The student completes the experiment at a concentration of 1 mol/dm3 and 3 mol/dm3.
It takes 148 seconds for the student to be unable to see the cross (X) when the reaction was carried out with a concentration of 3 mol/dm3.
Predict whether it will take a shorter or longer time for the student to be unable to see the cross when the reaction was carried out at a concentration of 1 mol/dm3. Use collision theory to explain your answer.
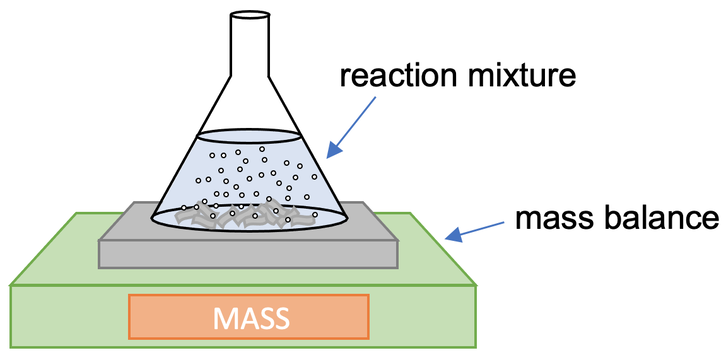
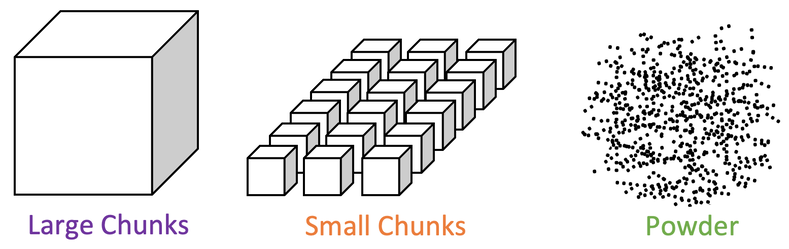
3) A student investigates how the surface area to volume ratio affects the rate of reaction between a given mass of calcium carbonate and some acid. The student sets up the apparatus like what is shown below.
The student measured the mass lost over time. He completes the reaction for 10 grams of calcium carbonate in three different shapes; large chunks, small chunks and powder. These different shapes are shown below.
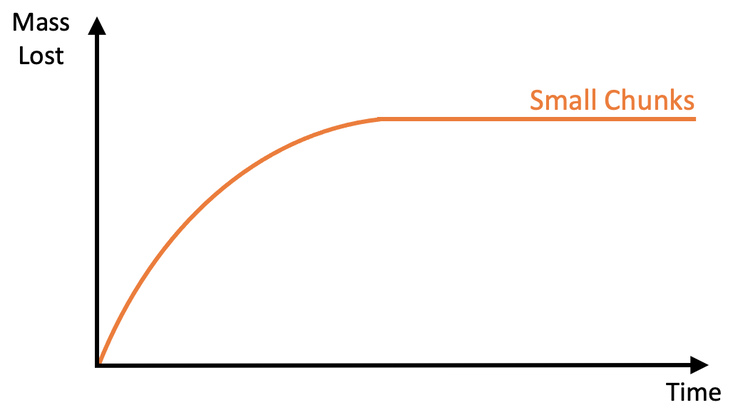
The student then draws a graph for mass lost against time for the small chunks. Their graph is shown below.
a)
i) Add a curve for the reaction with large chunks.
ii) Is the rate of reaction faster or slower? Use collision theory to explain your answer.
b) Add a curve for the reaction with powder.
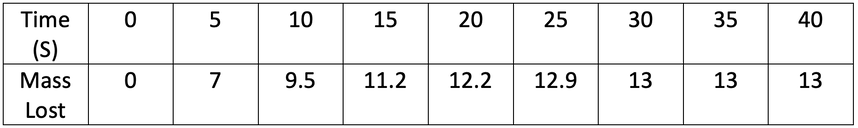
4) A student investigated the rate of reaction by measuring mass loss against time. His results are shown in the table below.
i) Add a curve for the reaction with large chunks.
ii) Is the rate of reaction faster or slower? Use collision theory to explain your answer.
b) Add a curve for the reaction with powder.
4) A student investigated the rate of reaction by measuring mass loss against time. His results are shown in the table below.
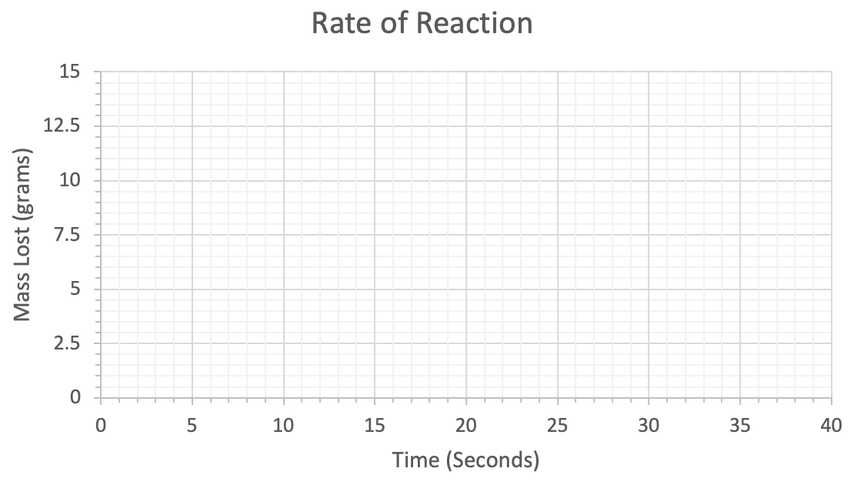
a) Plot his results on the graph below.
b) At what time does the reaction stop?
Keep this graph as we will be using it in the next quiz.
Keep this graph as we will be using it in the next quiz.