C7: Quiz 3 – Answers
a) The temperature
b) The volume of gas produced
c) Any two from the mass of magnesium strips, the concentration of acid, the volume of acid, the size/ shape/ surface area of the magnesium strips etc.
d)
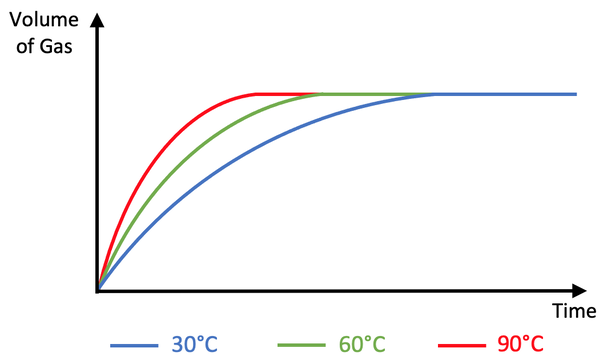
i) The rate of reaction is faster at 90°C. This is because at 90°C, the particles have more kinetic energy, which means that the particles move around faster. This results in more frequent collisions, which means that there is a greater rate of reaction (more gas is produced in a given period of time). Also, the particles having more kinetic energy means that more energy will be transferred during a collision, which is more likely to be greater than the activation energy thus meaning that reactions take place; this increases the rate of reaction.
Here is the graph for part d ii and part e
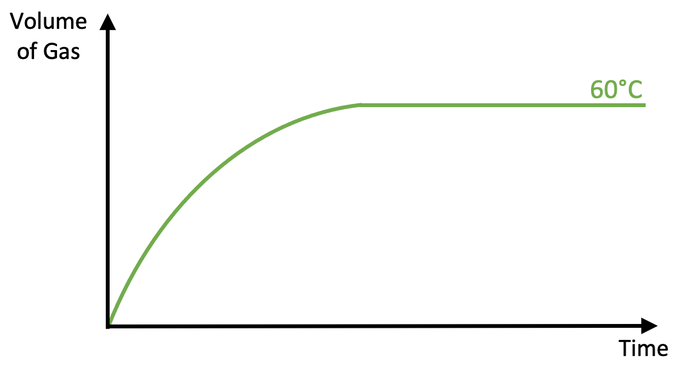
ii) A steeper curve becoming flat/ horizontal at the same maximum volume of gas being produced (the red curve on the graph above)
e) A flatter curve becoming flat/ horizontal at the same maximum volume of gas being produced (the blue curve on the graph above)
2) The time taken to be unable to see the cross at 1 mol/dm3 will be longer than the time for the concentration of 3 mol/dm3 (148 seconds). This is because a concentration of 1 mol/dm3 is a lower concentration than a concentration of 3 mol/dm3. This means that there are fewer particles in a given volume, which means that there will be less frequent collisions and therefore, a slower rate of reaction. A slower rate of reaction for this experiment means that the precipitate will form more slowly, thus meaning that it will take a longer time for us to be unable to see the cross underneath the flask
3)
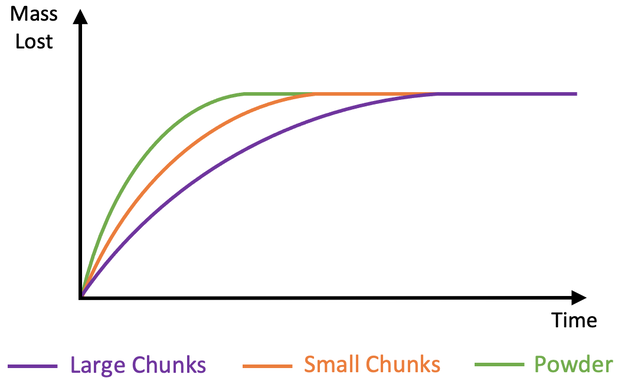
a) Here is the graph for this question.
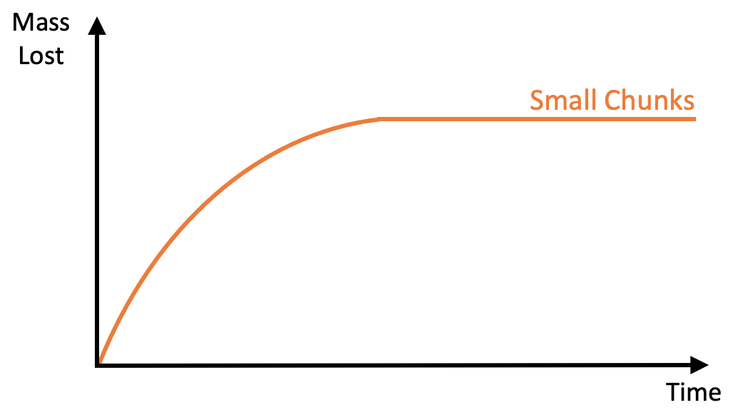
i) A flatter curve becoming flat/ horizontal at the same maximum mass lost (the purple line on the graph above)
ii) Larger chunks have a lower surface area to volume ratio compared to smaller chunks. This means that there is less surface area for the particles to collide upon, which means that there will be less frequent collisions and therefore a slower rate of reaction (mass is lost more slowly). This is why the graph is flatter
b) A steeper curve becoming flat/ horizontal at the same maximum mass lost (the green line on the graph above)
4)
a)
Click here for a printable PDF of the graphs in this quiz.
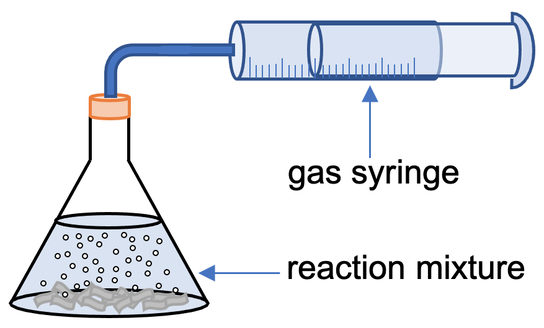
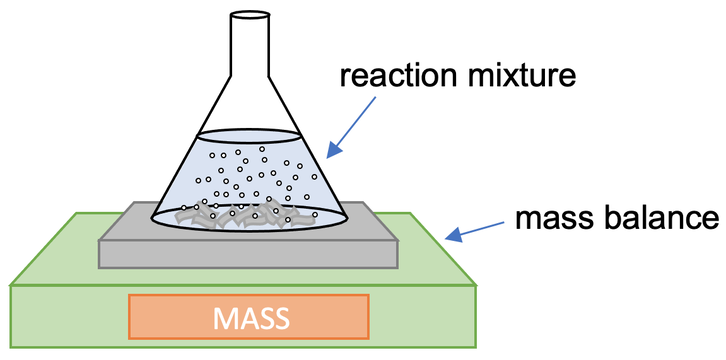
1) A student investigates how temperature affects the rate of reaction between a given mass of magnesium strips and some acid. The student sets up the apparatus like what is shown below.
a) What is the independent variable in this experiment?
b) What is the dependent variable in this experiment?
c) Give two variables that need to be controlled during this experiment.
The student then draws a graph for the volume of gas produced against time for the reaction at 60°C.
i) Is the rate of reaction at 90°C faster or slower than the rate of reaction at 60°C? Use collision theory to explain your answer.
ii) Add a curve to the above graph for the reaction at 90°C.
e) Add a curve to the above graph for the reaction at 30°C.
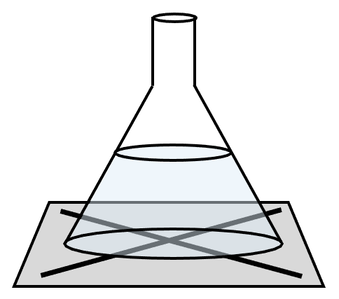
2) A student undertakes a precipitation reaction to see what effect concentration has on the rate of reaction. She sets up the apparatus like what is shown below.
The student completes the experiment at a concentration of 1 mol/dm3 and 3 mol/dm3.
It takes 148 seconds for the student to be unable to see the cross (X) when the reaction was carried out with a concentration of 3 mol/dm3.
Predict whether it will take a shorter or longer time for the student to be unable to see the cross when the reaction was carried out at a concentration of 1 mol/dm3. Use collision theory to explain your answer.
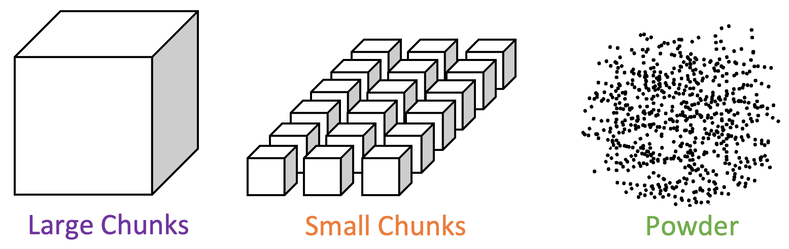
3) A student investigates how the surface area to volume ratio affects the rate of reaction between a given mass of calcium carbonate and some acid. The student sets up the apparatus like what is shown below.
i) Add a curve for the reaction with large chunks.
ii) Is the rate of reaction faster or slower? Use collision theory to explain your answer.
b) Add a curve for the reaction with powder.
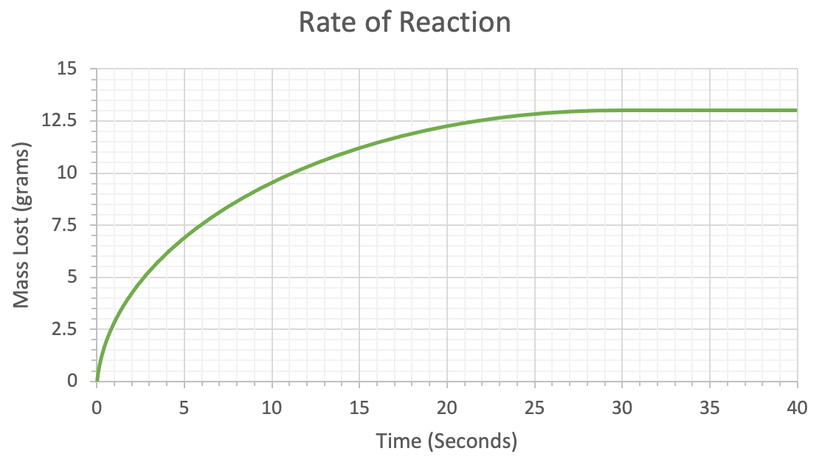
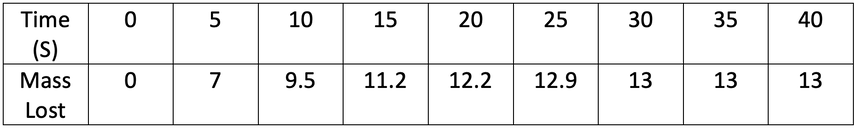
4) A student investigated the rate of reaction by measuring mass loss against time. His results are shown in the table below.
Keep this graph as we will be using it in the next quiz.